Ultra-high-performance liquid chromatography for the determination of exenatide in monkey plasma by tandem quadrupole mass spectrometry
- PMID: 29403823
- PMCID: PMC5760979
- DOI: 10.1016/j.jpha.2012.12.007
Ultra-high-performance liquid chromatography for the determination of exenatide in monkey plasma by tandem quadrupole mass spectrometry
Abstract
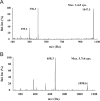
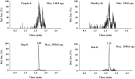
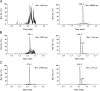
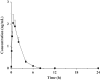
A highly sensitive ultra-high-performance liquid chromatographic-tandem mass spectrometry (UPLC/MS/MS) method was developed for the quantification of the synthetic peptide drug of exenatide in monkey plasma. Sample preparation was carried out by solid-phase extraction (SPE), and bivalirudin was used as the internal standard (IS). An excellent chromatographic separation was obtained on a reversed-phase C18 column with a gradient elution. Detection utilized a Qtrap 5500 system operated in the positive ion mode with multiple reaction monitoring (MRM). The proposed method was validated by assessing the specificity, linearity, intra- and inter-day precision and accuracy, recovery, and stability. The method resulted in a linear calibration range of 0.10-30 ng/mL, extracting with only 50 μL monkey plasma aliquots. The intra- and inter-day precisions (as relative standard deviation) were less than 7.5% and 9.6%, respectively. The method could be successfully utilized for the pharmacokinetic study of exenatide in monkeys following a single subcutaneous injection of Byetta.
Keywords: Exenatide; Monkey; Pharmacokinetics; Plasma; Quantitation; UPLC/MS/MS.
Figures

Similar articles
-
Simultaneous Determination of Purpurin, Munjistin and Mollugin in Rat Plasma by Ultra High Performance Liquid Chromatography-Tandem Mass Spectrometry: Application to a Pharmacokinetic Study after Oral Administration of Rubia cordifolia L. Extract.Molecules. 2016 Jun 1;21(6):717. doi: 10.3390/molecules21060717. Molecules. 2016. PMID: 27258244 Free PMC article.
-
[Determination of sixteen antibiotics and four β-agonists in human urine samples using ultra-performance liquid chromatography-tandem mass spectrometry based on high-throughput automatic solid-phase extraction].Se Pu. 2023 May 8;41(5):397-408. doi: 10.3724/SP.J.1123.2022.08025. Se Pu. 2023. PMID: 37087605 Free PMC article. Chinese.
-
Determination of fascaplysin in rat plasma with ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS): application to a pharmacokinetic study.J Pharm Biomed Anal. 2019 Jul 15;171:126-131. doi: 10.1016/j.jpba.2019.04.012. Epub 2019 Apr 8. J Pharm Biomed Anal. 2019. PMID: 30986762
-
[Determination of kojic acid in fermented foods by solid-phase extraction coupled with ultra performance liquid chromatography-tandem mass spectrometry].Se Pu. 2023 Jul;41(7):632-639. doi: 10.3724/SP.J.1123.2022.10002. Se Pu. 2023. PMID: 37387284 Free PMC article. Chinese.
-
A novel UPLC-MS/MS method for sensitive quantitation of boldine in plasma, a potential anti-inflammatory agent: application to a pharmacokinetic study in rats.Biomed Chromatogr. 2015 Mar;29(3):459-64. doi: 10.1002/bmc.3297. Epub 2014 Jul 26. Biomed Chromatogr. 2015. PMID: 25065486
Cited by
-
Ultra-sensitive bioanalysis of the therapeutic peptide exenatide for accurate pharmacokinetic analyses at effective plasma concentrations utilizing UPLC-MS/MS.J Pharm Anal. 2020 Jun;10(3):233-239. doi: 10.1016/j.jpha.2020.02.008. Epub 2020 Feb 22. J Pharm Anal. 2020. PMID: 32612869 Free PMC article.
-
Ultra-performance liquid chromatography electrospray ionization-tandem mass spectrometry method for the simultaneous determination of itraconazole and hydroxy itraconazole in human plasma.J Pharm Anal. 2014 Oct;4(5):316-324. doi: 10.1016/j.jpha.2013.09.005. Epub 2013 Sep 18. J Pharm Anal. 2014. PMID: 29403895 Free PMC article.
References
-
- Flatt P.R., Bailey C.J., Green B.D. Recent advances in antidiabetic drug therapies targeting the enteroinsular axis. Curr. Drug Metab. 2009;10:125–137. - PubMed
-
- Srinivasan B.T., Jarvis J., Khunti K. Recent advances in the management of type 2 diabetes mellitus: a review. Postgrad. Med. J. 2008;84:525–526. - PubMed
-
- Aroda V.R., Ratner R. The safety and tolerability of GLP-1 receptor agonists in the treatment of type 2 diabetes: a review. Diabetes Metab. Res. Rev. 2011;27:528–529. - PubMed
-
- Jones R. The safety and tolerability of GLP-1 receptor agonists in the treatment of type-2 diabetes. Int. J. Clin. Pract. 2010;64:1403–1414. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

