Bioanalytical method development and validation of milnacipran in rat plasma by LC-MS/MS detection and its application to a pharmacokinetic study
- PMID: 29403859
- PMCID: PMC5761007
- DOI: 10.1016/j.jpha.2013.03.009
Bioanalytical method development and validation of milnacipran in rat plasma by LC-MS/MS detection and its application to a pharmacokinetic study
Abstract
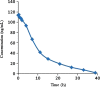
A simple, sensitive and specific liquid chromatography-tandem mass spectrometry (LC-MS/MS) method was developed for the quantification of milnacipran (MC) in rat plasma by using the liquid-liquid extraction method. Milnacipran-d10 (MCD10) was used as an internal standard (IS). Chromatographic separation was achieved on Zorbax SB-CN (4.6 mm×75 mm, 3.5 µm) column with an isocratic mobile phase composed of 10 mM ammonium acetate (pH 4.0) and methanol in the ratio of 25:75(v/v), at a flow-rate of 0.7 mL/min. MC and MCD10 were detected with proton adducts at m/z 247.2→230.3 and m/z 257.2→240.4 in multiple reaction monitoring (MRM) positive mode respectively. The method was validated over a linear concentration range of 1.00-400.00 ng/mL with a correlation coefficient (r2)≥0.9850. This method demonstrated intra- and inter-day precision within 5.40-10.85% and 4.40-8.29% and accuracy within 97.00-104.20% and 101.64-106.23%. MC was found to be stable throughout three freeze-thaw cycles, bench top and postoperative stability studies. This method was successfully applied to a pharmacokinetic study of rats through i.v. administration.
Keywords: LC–MS/MS; Milnacipran; Pharmacokinetics; Rat plasma.
Figures




Similar articles
-
Quantification of desloratadine in human plasma by LC-ESI-MS/MS and application to a pharmacokinetic study.J Pharm Anal. 2012 Jun;2(3):180-187. doi: 10.1016/j.jpha.2012.01.008. Epub 2012 Jan 31. J Pharm Anal. 2012. PMID: 29403740 Free PMC article.
-
A validated LC-MS/MS method for the determination of tolterodine and its metabolite in rat plasma and application to pharmacokinetic study.J Pharm Anal. 2013 Dec;3(6):489-499. doi: 10.1016/j.jpha.2013.04.005. Epub 2013 May 24. J Pharm Anal. 2013. PMID: 29403860 Free PMC article.
-
Development and validation of a sensitive bioanalytical method for the quantitative estimation of pantoprazole in human plasma samples by LC-MS/MS: application to bioequivalence study.J Chromatogr B Analyt Technol Biomed Life Sci. 2010 Jun 1;878(19):1499-505. doi: 10.1016/j.jchromb.2010.03.049. Epub 2010 Apr 9. J Chromatogr B Analyt Technol Biomed Life Sci. 2010. PMID: 20434410
-
Novel LC- ESI-MS/MS method for desvenlafaxine estimation human plasma: application to pharmacokinetic study.Biomed Chromatogr. 2016 Feb;30(2):249-55. doi: 10.1002/bmc.3542. Epub 2015 Aug 17. Biomed Chromatogr. 2016. PMID: 26095112
-
Development and validation of amisulpride in human plasma by HPLC coupled with tandem mass spectrometry and its application to a pharmacokinetic study.Sci Pharm. 2011 Jul-Sep;79(3):583-99. doi: 10.3797/scipharm.1105-12. Epub 2011 Jul 25. Sci Pharm. 2011. PMID: 21886905 Free PMC article.
Cited by
-
Gut microbe-derived milnacipran enhances tolerance to gut ischemia/reperfusion injury.Cell Rep Med. 2023 Mar 21;4(3):100979. doi: 10.1016/j.xcrm.2023.100979. Cell Rep Med. 2023. PMID: 36948152 Free PMC article.
-
Systemic and Brain Pharmacokinetics of Milnacipran in Mice: Comparison of Intraperitoneal and Intravenous Administration.Pharmaceutics. 2023 Dec 29;16(1):53. doi: 10.3390/pharmaceutics16010053. Pharmaceutics. 2023. PMID: 38258064 Free PMC article.
References
-
- Puozzo C., Pozet N., Deprez D. Pharmacokinetics of milnacipran in renal impairment. Eur. J. Drug Metab. Pharmacokinet. 1998;23(2):280–286. - PubMed
-
- Bantsiele G.B., Bentue-Ferrer D., Saïkali S. Behavioral effects of four antidepressants on an ischemic rat model of emotional disturbances. Behav. Brain Res. 2009;201(2):265–271. - PubMed
-
- Higuchi H., Yoshida K., Takahashi H. Milnacipran plasma levels and antidepressant response in Japanese major depressive patients. Hum. Psychopharmacol. 2003;18(4):255–259. - PubMed
-
- Rop P.P., Sournac M.H., Burle J. Blood concentration of milnacipran in a case of a fatal automobile accident. J. Anal. Toxicol. 2002;26(2):123–126. - PubMed
-
- Mandrioli R., Raggi M.A. Advances in the enantioseparation of second-generation antidepressant drugs by electrodriven methods. Electrophoresis. 2006;27(1):213–221. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

