Development of a sensitive and rapid method for quantitation of (S)-(-)- and (R)-(+)-metoprolol in human plasma by chiral LC-ESI-MS/MS
- PMID: 29403869
- PMCID: PMC5761053
- DOI: 10.1016/j.jpha.2013.02.008
Development of a sensitive and rapid method for quantitation of (S)-(-)- and (R)-(+)-metoprolol in human plasma by chiral LC-ESI-MS/MS
Abstract
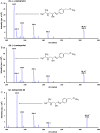
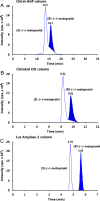
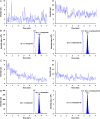
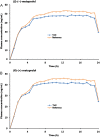
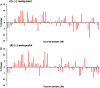
A selective, sensitive and high throughput liquid chromatography-tandem mass spectrometry (LC-ESI-MS/MS) method has been developed for separation and quantification of metoprolol enantiomers on a chiral Lux Amylose-2 (250 mm×4.6 mm, 5 μm) column. Solid phase extraction of (S)-(-)- and (R)-(+)-metoprolol and rac-metoprolol-d6 as an internal standard (IS) was achieved on Lichrosep DVB HL cartridges employing 200 μL human plasma. Both the analytes were chromatographically separated with a resolution factor of 2.24 using 15 mM ammonium acetate in water, pH 5.0 and 0.1% (v/v) diethyl amine in acetonitrile (50:50, v/v) as the mobile phase within 7.0 min. The precursor→product ion transitions for the enantiomers and IS were monitored in the multiple reaction monitoring and positive ionization mode. The method was validated over the concentration range of 0.500-500 ng/mL for both the enantiomers. Matrix effect was assessed by post-column analyte infusion experiment and the mean extraction recovery was greater than 94.0% for both the enantiomers at all quality control levels. The stability of analytes was evaluated in plasma and whole blood under different storage conditions. The method was successfully applied to a clinical study in 14 healthy volunteers after oral administration of 200 mg metoprolol tablet under fasting conditions. The assay reproducibility is shown by reanalysis of 68 incurred samples. The suitability of the developed method was assessed in comparison with different chromatographic methods developed for stereoselective analysis of metoprolol in biological matrices.
Keywords: Chiral column; Chromatographic separation; Human plasma; LC–ESI–MS/MS; R-(+)-metoprolol; S-(−)-metoprolol.
Figures

References
-
- Liu K., Zhong D., Chen X. Enantioselective quantification of chiral drugs in human plasma with LC-MS/MS. Bioanalysis. 2009;1:561–576. - PubMed
-
- Aturki Z., D'Orazio G., Rocco A. Advances in the enantioseparation of β-blocker drugs by capillary electromigration techniques. Electrophoresis. 2011;32:2602–2628. - PubMed
-
- Bristow M.R. Beta-adrenergic receptor blockade in chronic heart failure. Circulation. 2000;101:558–569. - PubMed
-
- Shrivastav P.S., Buha S.M., Sanyal M. Detection and quantitation of β-blockers in plasma and urine. Bioanalysis. 2010;2:263–276. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

