Selective separation, detection of zotepine and mass spectral characterization of degradants by LC-MS/MS/QTOF
- PMID: 29403872
- PMCID: PMC5761085
- DOI: 10.1016/j.jpha.2013.04.002
Selective separation, detection of zotepine and mass spectral characterization of degradants by LC-MS/MS/QTOF
Abstract
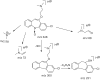
A simple, precise, accurate stability-indicating gradient reversed-phase high-performance liquid chromatographic (RP-HPLC) method was developed for the quantitative determination of zotepine (ZTP) in bulk and pharmaceutical dosage forms in the presence of its degradation products (DPs). The method was developed using Phenomenex C18 column (250 mm×4.6 mm i.d., 5 µm) with a mobile phase containing a gradient mixture of solvents, A (0.05% trifluoroacetic acid (TFA), pH=3.0) and B (acetonitrile). The eluted compounds were monitored at 254 nm; the run time was within 20.0 min, in which ZTP and its DPs were well separated, with a resolution of >1.5. The stress testing of ZTP was carried out under acidic, alkaline, neutral hydrolysis, oxidative, photolytic and thermal stress conditions. ZTP was found to degrade significantly in acidic, photolytic, thermal and oxidative stress conditions and remain stable in basic and neutral conditions. The developed method was validated with respect to specificity, linearity, limit of detection, limit of quantification, accuracy, precision and robustness as per ICH guidelines. This method was also suitable for the assay determination of ZTP in pharmaceutical dosage forms. The DPs were characterized by LC-MS/MS and their fragmentation pathways were proposed.
Keywords: Bulk drugs and formulations; Characterization; ESI-Q-TOF-MS; Stability-indicating RP–HPLC method; Zotepine.
Figures








Similar articles
-
Structural characterization of alkaline and oxidative stressed degradation products of lurasidone using LC/ESI/QTOF/MS/MS.J Pharm Biomed Anal. 2015 Feb;105:1-9. doi: 10.1016/j.jpba.2014.11.035. Epub 2014 Nov 26. J Pharm Biomed Anal. 2015. PMID: 25527975
-
Selective separation and characterisation of stress degradation products and process impurities of prucalopride succinate by LC-QTOF-MS/MS.J Pharm Biomed Anal. 2016 Jun 5;125:219-28. doi: 10.1016/j.jpba.2016.03.047. Epub 2016 Mar 24. J Pharm Biomed Anal. 2016. PMID: 27037978
-
Stability-indicating UPLC method for determination of Valsartan and their degradation products in active pharmaceutical ingredient and pharmaceutical dosage forms.J Pharm Biomed Anal. 2010 Nov 2;53(3):483-9. doi: 10.1016/j.jpba.2010.05.022. J Pharm Biomed Anal. 2010. PMID: 20646890
-
Development and validation of a novel stability-indicating HPLC method for the quantitative determination of eleven related substances in ezetimibe drug substance and drug product.Talanta. 2015 Jul 1;139:67-74. doi: 10.1016/j.talanta.2015.02.039. Epub 2015 Feb 27. Talanta. 2015. PMID: 25882410
-
UPLC and LC-MS studies on degradation behavior of irinotecan hydrochloride and development of a validated stability-indicating ultra-performance liquid chromatographic method for determination of irinotecan hydrochloride and its impurities in pharmaceutical dosage forms.J Chromatogr Sci. 2012 Oct;50(9):810-9. doi: 10.1093/chromsci/bms075. Epub 2012 Jun 1. J Chromatogr Sci. 2012. PMID: 22661461
Cited by
-
Studies on photodegradation process of psychotropic drugs: a review.Environ Sci Pollut Res Int. 2017 Jan;24(2):1152-1199. doi: 10.1007/s11356-016-7727-5. Epub 2016 Sep 30. Environ Sci Pollut Res Int. 2017. PMID: 27696160 Free PMC article. Review.
References
-
- ICH, Stability Testing of New Drug Substances and Products Q1A (R2), in: Proceedings of the International Conference on Harmonization, IFPMA, Geneva, 2003.
-
- WHO . World Health Organization; Geneva: 2007. Draft Stability Testing of Active Pharmaceutical Ingredients and Pharmaceutical Products.
-
- CPMP, Note for Guidance on Stability Testing: Stability Testing of Existing Active Substances and Related Finished Products, Committee for Proprietary Medicinal Products, EMEA, London, 2002.
-
- TPD, Guidance for Industry Stability Testing of Existing Drug Substances and Products, Therapeutic Products Directorate, Health Canada, Ottawa, ON, 2003.
-
- Singh S., Bakshi M. Guidance on conduct of stress tests to determine inherent stability of drugs. Pharm. Tech. On-line. 2000;24:1–14. http://xa.yimg.com/kq/groups/2299115/609207339/name/Guidance_Stress_Test....
LinkOut - more resources
Full Text Sources
Other Literature Sources

