Improved simultaneous quantitation of candesartan and hydrochlorthiazide in human plasma by UPLC-MS/MS and its application in bioequivalence studies
- PMID: 29403876
- PMCID: PMC5761088
- DOI: 10.1016/j.jpha.2013.05.003
Improved simultaneous quantitation of candesartan and hydrochlorthiazide in human plasma by UPLC-MS/MS and its application in bioequivalence studies
Abstract
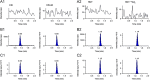
A validated ultra-performance liquid chromatography mass spectrometric method (UPLC-MS/MS) was used for the simultaneous quantitation of candesartan (CN) and hydrochlorothiazide (HCT) in human plasma. The analysis was performed on UPLC-MS/MS system using turbo ion spray interface. Negative ions were measured in multiple reaction monitoring (MRM) mode. The analytes were extracted using a liquid-liquid extraction (LLE) method by using 0.1 mL of plasma volume. The lower limit of quantitation for CN and HCT was 1.00 ng/mL whereas the upper limit of quantitation was 499.15 ng/mL and 601.61 ng/mL for CN and HCT respectively. CN d4 and HCT-13Cd2 were used as the internal standards for CN and HCT respectively. The chromatography was achieved within 2.0 min run time using a C18 Phenomenex, Gemini NX (100 mm×4.6 mm, 5 µm) column with organic mixture:buffer solution (80:20, v/v) at a flow rate of 0.800 mL/min. The method has been successfully applied to establish the bioequivalence of candesartan cilexetil (CNC) and HCT immediate release tablets with reference product in human subjects.
Keywords: Bioequivalence; Candesartan cilexetil; Candesartan cilexetil-hydrochlorothiazide (ATACAND HCT); Hydrochlorothiazide; UPLC–MS/MS.
Figures


References
-
- Gao F., Zhang Z., Bu H. Nanoemulsion improves the oral absorption of candesartan cilexetil in rats: performance and mechanism. J. Controlled Release. 2011;149:168–174. - PubMed
-
- Lee H.Y., Hong B.K., Chung W.J. Phase IV, 8-week, multicenter, randomized, active treatment–controlled, parallel group, efficacy, and tolerability study of high-dose candesartan cilexetil combined with hydrochlorothiazide in Korean adults with stage II hypertension. Clin. Ther. 2011;33:1043–1056. - PubMed
-
- Campbell M., Sonkodi S., Soucek M. A candesartan cilexetil/hydrochlorothiazide combination tablet provides effective blood pressure control in hypertensive patients inadequately controlled on monotherapy. Clin. Exp. Hypertens. 2001;23:345–355. - PubMed
-
- Bönner G., Fuchs W. Fixed combination of candesartan with hydrochlorothiazide in patients with severe primary hypertension. Curr. Med. Res. Opin. 2004;20:597–602. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

