Stability-indicating HPLC-DAD methods for determination of two binary mixtures: Rabeprazole sodium-mosapride citrate and rabeprazole sodium-itopride hydrochloride
- PMID: 29403889
- PMCID: PMC5761220
- DOI: 10.1016/j.jpha.2013.09.009
Stability-indicating HPLC-DAD methods for determination of two binary mixtures: Rabeprazole sodium-mosapride citrate and rabeprazole sodium-itopride hydrochloride
Abstract
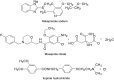
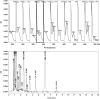
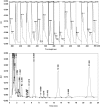
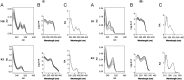
Two selective stability-indicating HPLC methods are described for determination of rabeprazole sodium (RZ)-mosapride citrate (MR) and RZ-itopride hydrochloride (IO) mixtures in the presence of their ICH-stress formed degradation products. Separations were achieved on X-Bridge C18 column using two mobile phases: the first for RZ-MR mixture consisted of acetonitrile: 0.025 M KH2PO4 solution: TEA (30:69:1 v/v; pH 7.0); the second for RZ-IO mixture was at ratio of 25:74:1 (v/v; pH 9.25). The detection wavelength was 283 nm. The two methods were validated and validation acceptance criteria were met in all cases. Peak purity testing using contrast angle theory, relative absorbance and log A versus the wavelengths plots were presented. The % recoveries of the intact drugs were between 99.1% and 102.2% with RSD% values less than 1.6%. Application of the proposed HPLC methods indicated that the methods could be adopted to follow the stability of their formulations.
Keywords: Itopride hydrochloride; Mosapride citrate; Peak purity; Rabeprazole sodium; Stability-indicating HPLC–DAD.
Figures


References
-
- S.C. Sweetman, Martindale the Complete Drug Reference, 35th ed., Pharmaceutical Press, London, 2007, pp. 1566, 1577, 1590.
-
- Carswel C.I., Goa K.L. Rabeprazole: an update of its use in acid-related disorders. Drugs. 2001;61:2327–2356. - PubMed
-
- Prasanna B.R., Reddy M.S. Development and validation of RP-HPLC for the determination of rabeprazole sodium in pharmaceutical formulations and human plasma. Asian J. Res. Chem. 2009;2(1):49–51.
-
- Garcia C.V., Paim C.S., Steppe M. Development and validation of a dissolution test for rabeprazole sodium in coated tablets. J. Pharm. Biomed. Anal. 2006;41(3):833–837. - PubMed
-
- Garcia C.V., Paim C.S., Steppe M. New liquid chromatographic method for determination of rabeprazole sodium in coated tablets. J. AOAC Int. 2004;87(4):842–846. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

