Simultaneous determination of amlodipine, valsartan and hydrochlorothiazide by LC-ESI-MS/MS and its application to pharmacokinetics in rats
- PMID: 29403906
- PMCID: PMC5761358
- DOI: 10.1016/j.jpha.2013.12.003
Simultaneous determination of amlodipine, valsartan and hydrochlorothiazide by LC-ESI-MS/MS and its application to pharmacokinetics in rats
Abstract
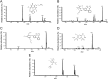
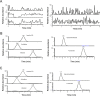
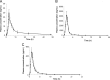
Polypill is a fixed-dose combination that contains three or more active ingredients used as a single daily pill to achieve a large effect in preventing cardiovascular disease with minimal adverse effects. A novel and accurate liquid chromatography tandem mass spectrometry method using electrospray ionization mode has been developed and validated for the simultaneous determination of amlodipine (AMD), valsartan (VAL) using losartan (LOS) as an internal standard (IS), and hydrochlorothiazide (HCT) using furosemide (FSD) as an IS. The separation was carried on Aquasil C18 (50 mm×2.1 mm, 5 µm) reversed phase column using acetonitrile and water containing 0.1% formic acid (50:50, v/v) as the mobile phase. The method was validated in terms of linearity, accuracy and precision over the concentration range of 1-1000 ng/mL. The intra and inter-day precision and accuracy, stability and extraction recoveries of all the analytes were in the acceptable range. This method can be successfully applied to the pharmacokinetic study of AMD, VAL and HCT when given as a polypill.
Keywords: Amlodipine; Exforge HCT; Hydrochlorothiazide; Polypill; Valsartan.
Figures
Similar articles
-
Application of an LC-MS/MS method for the analysis of amlodipine, valsartan and hydrochlorothiazide in polypill for a bioequivalence study.J Pharm Anal. 2017 Oct;7(5):309-316. doi: 10.1016/j.jpha.2017.06.001. Epub 2017 Jun 4. J Pharm Anal. 2017. PMID: 29404054 Free PMC article.
-
Reliable and easy-to-use LC-MS/MS-method for simultaneous determination of the antihypertensives metoprolol, amlodipine, canrenone and hydrochlorothiazide in patients with therapy-refractory arterial hypertension.J Pharm Biomed Anal. 2019 Feb 5;164:373-381. doi: 10.1016/j.jpba.2018.11.002. Epub 2018 Nov 5. J Pharm Biomed Anal. 2019. PMID: 30439665
-
Simultaneous Determination of a Fixed-Dose Combination of Lercanidipine and Valsartan in Human Plasma by LC-MS-MS: Application to a Pharmacokinetic Study.J Chromatogr Sci. 2016 Oct 17;54(9):1553-1559. doi: 10.1093/chromsci/bmw102. J Chromatogr Sci. 2016. PMID: 27405507
-
Development and Validation of a LC-MS/MS Method for the Simultaneous Estimation of Amlodipine and Valsartan in Human Plasma: Application to a Bioequivalence Study.Sci Pharm. 2014 Mar 26;82(3):585-600. doi: 10.3797/scipharm.1402-11. Print 2014 Jul-Sep. Sci Pharm. 2014. PMID: 25853070 Free PMC article.
-
Simple RP-HPLC method for determination of triple drug combination of valsartan, amlodipine and hydrochlorothiazide in human plasma.Acta Pharm. 2012 Mar;62(1):45-58. doi: 10.2478/v10007-012-0004-3. Acta Pharm. 2012. PMID: 22472448
Cited by
-
Application of an LC-MS/MS method for the analysis of amlodipine, valsartan and hydrochlorothiazide in polypill for a bioequivalence study.J Pharm Anal. 2017 Oct;7(5):309-316. doi: 10.1016/j.jpha.2017.06.001. Epub 2017 Jun 4. J Pharm Anal. 2017. PMID: 29404054 Free PMC article.
-
Simultaneous quantification of amiloride and hydrochlorothiazide in human plasma by liquid chromatography-tandem mass spectrometry.J Pharm Anal. 2017 Oct;7(5):288-296. doi: 10.1016/j.jpha.2017.03.007. Epub 2017 Mar 24. J Pharm Anal. 2017. PMID: 29404051 Free PMC article.
-
Dispersive Solid Phase Extraction Using Magnetic Nanoparticles Performed in a Narrow-Bored Tube for Extraction of Atorvastatin, Losartan, and Valsartan in Plasma.Adv Pharm Bull. 2019 Feb;9(1):138-146. doi: 10.15171/apb.2019.017. Epub 2019 Feb 21. Adv Pharm Bull. 2019. PMID: 31011568 Free PMC article.
-
Simultaneous Determination and Pharmacokinetic Study of Losartan, Losartan Carboxylic Acid, Ramipril, Ramiprilat, and Hydrochlorothiazide in Rat Plasma by a Liquid Chromatography/Tandem Mass Spectrometry Method.Sci Pharm. 2014 Nov 30;83(1):107-24. doi: 10.3797/scipharm.1410-15. Print 2015 Jan-Mar. Sci Pharm. 2014. PMID: 26839805 Free PMC article.
-
Recent Advances in Liquid Chromatography-Mass Spectrometry (LC-MS) Applications in Biological and Applied Sciences.Anal Sci Adv. 2025 Jun 27;6(1):e70024. doi: 10.1002/ansa.70024. eCollection 2025 Jun. Anal Sci Adv. 2025. PMID: 40584301 Free PMC article. Review.
References
-
- Ramani A.V., Senguptha P., Mullangi R. Development and validation of a highly sensitive and robust LC–ESI-MS/MS method for simultaneous quantitation of simvastatin acid, amlodipine and valsartan in human plasma: application to a clinical pharmacokinetic study. Biomed. Chromatogr. 2009;23:615–622. - PubMed
-
- Wellington K., Faulds D.M. Valsartan/hydrochlorothiazide: a review of its pharmacology, therapeutic efficacy and place in the management of hypertension. Drugs. 2002;62(13):1983–2005. - PubMed
-
- Crystal Phend, Staff Writer, FDA Approves Triple-Drug Antihypertensive Polypill, MedPageToday,Washington, (〈http://www.medpagetoday.com/ProductAlert/Prescriptions/14032〉), May 1 2009.
-
- Safety Labeling Changes Approved By FDA Center for Drug Evaluation and Research(CDER), Exforge HCT (amlodipine/valsartan/hydrochlorothiazide) Tablets, (〈http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm262227.htm〉), 2012.
-
- H.C.T. Exforge, Rx List, (〈http://www.rxlist.com/exforge-hct-drug.htm〉), September 18 2013.
LinkOut - more resources
Full Text Sources
Other Literature Sources

