Determination of cilostazol and its active metabolite 3,4-dehydro cilostazol from small plasma volume by UPLC-MS/MS
- PMID: 29403909
- PMCID: PMC5761466
- DOI: 10.1016/j.jpha.2014.08.001
Determination of cilostazol and its active metabolite 3,4-dehydro cilostazol from small plasma volume by UPLC-MS/MS
Abstract
A simple, rapid and sensitive ultra performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) method has been developed for the simultaneous determination of cilostazol and its pharmacologically active metabolite 3,4-dehydro cilostazol in human plasma using deuterated analogs as internal standards (ISs). Plasma samples were prepared using solid phase extraction and chromatographic separation was performed on UPLC BEH C18 (50 mm×2.1 mm, 1.7 µm) column. The method was established over a concentration range of 0.5-1000 ng/mL for cilostazol and 0.5-500 ng/mL for 3,4-dehydro cilostazol. Intra- and inter-batch precision (% CV) and accuracy for the analytes were found within 0.93-1.88 and 98.8-101.7% for cilostazol and 0.91-2.79 and 98.0-102.7% for the metabolite respectively. The assay recovery was within 95-97% for both the analytes and internal standards. The method was successfully applied to support a bioequivalence study of 100 mg cilostazol in 30 healthy subjects.
Keywords: 3,4-dehydro cilostazol; Cilostazol; High throughput; Sensitive; UPLC−MS/MS.
Figures

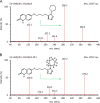
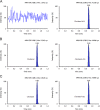
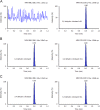
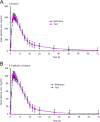

Similar articles
-
UPLC-MS/MS assay for the simultaneous quantification of carvedilol and its active metabolite 4'-hydroxyphenyl carvedilol in human plasma to support a bioequivalence study in healthy volunteers.Biomed Chromatogr. 2013 Aug;27(8):974-86. doi: 10.1002/bmc.2889. Epub 2013 Mar 11. Biomed Chromatogr. 2013. PMID: 23483571 Clinical Trial.
-
Determination of lercanidipine in human plasma by an improved UPLC-MS/MS method for a bioequivalence study.J Pharm Anal. 2016 Apr;6(2):87-94. doi: 10.1016/j.jpha.2015.09.001. Epub 2015 Sep 28. J Pharm Anal. 2016. PMID: 29403967 Free PMC article.
-
Liquid chromatography - tandem mass spectrometry for the simultaneous quantitation of glipizide, cilostazol and its active metabolite 3, 4-dehydro-cilostazol in rat plasma: application for a pharmacokinetic study.Arzneimittelforschung. 2012 Sep;62(9):425-32. doi: 10.1055/s-0032-1316374. Epub 2012 Jul 20. Arzneimittelforschung. 2012. PMID: 22821721
-
A new simple method for quantification of cilostazol and its active metabolite in human plasma by LC-MS/MS: Application to pharmacokinetics of cilostazol associated with CYP genotypes in healthy Chinese population.Biomed Chromatogr. 2021 Oct;35(10):e5150. doi: 10.1002/bmc.5150. Epub 2021 May 25. Biomed Chromatogr. 2021. PMID: 33894005
-
Development and validation of a liquid chromatography/tandem mass spectrometry assay for the simultaneous determination of nateglinide, cilostazol and its active metabolite 3,4-dehydro-cilostazol in Wistar rat plasma and its application to pharmacokinetic study.J Chromatogr B Analyt Technol Biomed Life Sci. 2008 Apr 1;865(1-2):91-8. doi: 10.1016/j.jchromb.2008.02.013. Epub 2008 Feb 23. J Chromatogr B Analyt Technol Biomed Life Sci. 2008. PMID: 18342586
Cited by
-
Effect of Baicalein on the Pharmacokinetics of Cilostazol and Its Two Metabolites in Rat Plasma Using UPLC-MS/MS Method.Front Pharmacol. 2022 Apr 27;13:888054. doi: 10.3389/fphar.2022.888054. eCollection 2022. Front Pharmacol. 2022. PMID: 35571101 Free PMC article.
References
-
- US Department of Health and Human Services, Food and Drug Administration. NME Drug and New Biological Approval in 1999. Available from: 〈http://www.fda.gov/…/howdrugsaredevelopedandapproved/drugandbiologicapp... (Last accessed April, 2014).
-
- Al-Qudah Z.A., Hassan A.E., Qureshi A.L. Cilostazol in patients with ischemic stroke. Expert. Opin. Pharmacother. 2011;12:1305–1315. - PubMed
-
- Liu Y., Shaku Y., Kambayashi J. Phosphodi Esterases as Drug Targets. In: Francis S.H., Conti M., Houslay M.D., editors. Hand-book of Experimental Pharmacology. Springer-Verlag; Berlin, Germany: 2011. p. 211.
-
- Liu Y., Shaku Y., Yoshitak M. Cilostazol (Pletal®): a dual inhibitor of cyclic nucleotide phosphodiesterase type 3 and adenosine uptake. Cardiovasc. Drug Rev. 2001;19:369–386. - PubMed
-
- Hiratsuka M., Hinai Y., Sasaki T. Characterization of human cytochrome P450 enzymes involved in the metabolism of cilostazol. Drug Metab. Dispos. 2007;35:1730–1732. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

