Selective and rapid determination of raltegravir in human plasma by liquid chromatography-tandem mass spectrometry in the negative ionization mode
- PMID: 29403921
- PMCID: PMC5761471
- DOI: 10.1016/j.jpha.2014.10.002
Selective and rapid determination of raltegravir in human plasma by liquid chromatography-tandem mass spectrometry in the negative ionization mode
Abstract
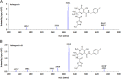
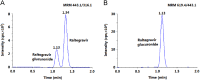
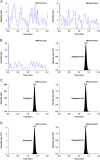
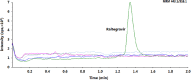
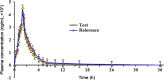
A selective and rapid high-performance liquid chromatography-tandem mass spectrometry method was developed and validated for the quantification of raltegravir using raltegravir-d3 as an internal standard (IS). The analyte and IS were extracted with methylene chloride and n-hexane solvent mixture from 100 µL human plasma. The chromatographic separation was achieved on a Chromolith RP-18e endcapped C18 (100 mm×4.6 mm) column in a run time of 2.0 min. Quantitation was performed in the negative ionization mode using the transitions of m/z 443.1→316.1 for raltegravir and m/z 446.1→319.0 for IS. The linearity of the method was established in the concentration range of 2.0-6000 ng/mL. The mean extraction recovery for raltegravir and IS was 92.6% and 91.8%, respectively, and the IS-normalized matrix factors for raltegravir ranged from 0.992 to 0.999. The application of this method was demonstrated by a bioequivalence study on 18 healthy subjects.
Keywords: Bioequivalence study; Human plasma; LC–ESI–MS/MS; Negative ionization mode; Raltegravir.
Figures

References
-
- Merck Sharp & Dohme Corporation, ISENTRESS® (raltegravir) oral tablets. Prescribing Information, Whitehouse Station, NJ 08889, USA, April 2012. 〈https://www.merck.com/product/usa/pi_circulars/i/isentress/isentress_pi...
LinkOut - more resources
Full Text Sources
Other Literature Sources

