Monolithic LC method applied to fesoterodine fumarate low dose extended-release tablets: Dissolution and release kinetics
- PMID: 29403925
- PMCID: PMC5761478
- DOI: 10.1016/j.jpha.2014.10.001
Monolithic LC method applied to fesoterodine fumarate low dose extended-release tablets: Dissolution and release kinetics
Abstract
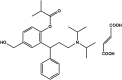
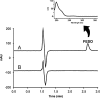
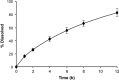
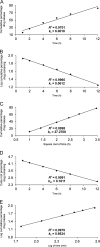
A dissolution test for fesoterodine low dose extended-release tablets using liquid chromatographic (LC) method equipped with a C18 monolithic column was developed and validated. LC system was operated isocratically at controlled temperature (40 °C) using a mobile phase of acetonitrile:methanol:0.03 M ammonium acetate (pH 3.8) (30:15:55, v/v/v), run at a flow rate of 1.5 mL/min and detected at 208 nm. The best dissolution conditions for this formulation were achieved using a USP apparatus 2 (paddle) at 100 rpm and 900 mL of phosphate buffer at pH 6.8 as the dissolution medium. Validation parameters such as the specificity, linearity, accuracy, precision, and robustness were evaluated according to international guidelines, giving results within the acceptable range. The kinetic parameters of drug release were also investigated using model-dependent methods and the dissolution profiles were best described by the Higuchi model. The validated dissolution test can be applied for quality control of this formulation.
Keywords: Dissolution test; Fesoterodine; Low dose extended-release tablets; Monolithic LC; Release kinetics.
Figures

Similar articles
-
Development and validation of a stability-indicating LC method for the determination of venlafaxine in extended-release capsules and dissolution kinetic studies.J Chromatogr Sci. 2009 Oct;47(9):770-6. doi: 10.1093/chromsci/47.9.770. J Chromatogr Sci. 2009. PMID: 19835686
-
Liquid chromatographic determination of norfloxacin in extended-release tablets.J Chromatogr Sci. 2009 Oct;47(9):739-44. doi: 10.1093/chromsci/47.9.739. J Chromatogr Sci. 2009. PMID: 19835680
-
Dissolution method for delapril and manidipine combination tablets based on an absorption profile of manidipine.J Pharm Anal. 2016 Feb;6(1):49-55. doi: 10.1016/j.jpha.2015.10.002. Epub 2015 Oct 23. J Pharm Anal. 2016. PMID: 29403962 Free PMC article.
-
LC determination of entacapone in tablets: in vitro dissolution studies.J Chromatogr Sci. 2010 Oct;48(9):755-9. doi: 10.1093/chromsci/48.9.755. J Chromatogr Sci. 2010. PMID: 20875238
-
Development and validation of new discriminative dissolution method for carvedilol tablets.Indian J Pharm Sci. 2011 Sep;73(5):527-36. doi: 10.4103/0250-474X.99000. Indian J Pharm Sci. 2011. PMID: 22923865 Free PMC article.
Cited by
-
Automatic Dissolution Testing with High-Temporal Resolution for Both Immediate-Release and Fixed-Combination Drug Tablets.Sci Rep. 2019 Nov 19;9(1):17114. doi: 10.1038/s41598-019-53750-w. Sci Rep. 2019. PMID: 31745201 Free PMC article.
-
Regulation of drug release performance using mixed doxorubicin-doxorubicin dimer nanoparticles as a pH-triggered drug self-delivery system.J Pharm Anal. 2022 Feb;12(1):122-128. doi: 10.1016/j.jpha.2021.03.001. Epub 2021 Mar 9. J Pharm Anal. 2022. PMID: 35573875 Free PMC article.
References
-
- Costa P., Lobo J.M.S. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001;13:123–133. - PubMed
-
- Dash S., Murthy P.N., Nath L. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. Drug Res. 2010;67:217–223. - PubMed
-
- Gao Z. Mathematical modeling of variables involved in dissolution testing. J. Pharm. Sci. 2011;100:4934–4942. - PubMed
-
- Malhotra B., Guan Z., Wood N. Pharmacokinetic profile of fesoterodine. Int. J. Clin. Pharmacol. Ther. 2008;46:556–563. - PubMed
-
- Chapple C.R., Khullar V., Gabriel Z. The effects of antimuscarinic treatments in overactive bladder: an update of a systematic review and meta-analysis. Eur. Urol. 2008;54:543–562. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

