Significance and challenges of stereoselectivity assessing methods in drug metabolism
- PMID: 29403956
- PMCID: PMC5762452
- DOI: 10.1016/j.jpha.2015.12.004
Significance and challenges of stereoselectivity assessing methods in drug metabolism
Abstract
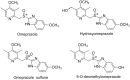
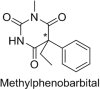
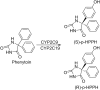
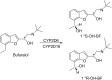
Stereoselectivity in drug metabolism can not only influence the pharmacological activities, tolerability, safety, and bioavailability of drugs directly, but also cause different kinds of drug-drug interactions. Thus, assessing stereoselectivity in drug metabolism is of great significance for pharmaceutical research and development (R&D) and rational use in clinic. Although there are various methods available for assessing stereoselectivity in drug metabolism, many of them have shortcomings. The indirect method of chromatographic methods can only be applicable to specific samples with functional groups to be derivatized or form complex with a chiral selector, while the direct method achieved by chiral stationary phases (CSPs) is expensive. As a detector of chromatographic methods, mass spectrometry (MS) is highly sensitive and specific, whereas the matrix interference is still a challenge to overcome. In addition, the use of nuclear magnetic resonance (NMR) and immunoassay in chiral analysis are worth noting. This review presents several typical examples of drug stereoselective metabolism and provides a literature-based evaluation on current chiral analytical techniques to show the significance and challenges of stereoselectivity assessing methods in drug metabolism.
Keywords: Capillary electrophoresis; Chiral chromatography; Enantiomer; Immunoassay; Mass spectrometry; NMR.
Figures

Similar articles
-
[Advances in chiral separation and analysis by capillary electrophoresis-mass spectrometry].Se Pu. 2022 Jun;40(6):509-519. doi: 10.3724/SP.J.1123.2021.11006. Se Pu. 2022. PMID: 35616196 Free PMC article. Review. Chinese.
-
[Research progress of molecularly imprinted polymers in separation of chiral drugs by capillary electrochromatography].Se Pu. 2020 Sep 8;38(9):1046-1056. doi: 10.3724/SP.J.1123.2020.03018. Se Pu. 2020. PMID: 34213271 Review. Chinese.
-
Screening of synthetic PDE-5 inhibitors and their analogues as adulterants: analytical techniques and challenges.J Pharm Biomed Anal. 2014 Jan;87:176-90. doi: 10.1016/j.jpba.2013.04.037. Epub 2013 May 6. J Pharm Biomed Anal. 2014. PMID: 23721687 Review.
-
Bioactivity and analysis of chiral compounds.Arh Hig Rada Toksikol. 2000 Sep;51(3):335-41. Arh Hig Rada Toksikol. 2000. PMID: 11148938
-
Enantiomer separation and indirect chromatographic absolute configuration prediction of chiral pirinixic acid derivatives: Limitations of polysaccharide-type chiral stationary phases in comparison to chiral anion-exchangers.J Chromatogr A. 2010 Feb 12;1217(7):1033-40. doi: 10.1016/j.chroma.2009.10.048. Epub 2009 Oct 21. J Chromatogr A. 2010. PMID: 19896137
Cited by
-
An ultra-sensitive and easy-to-use assay for sensing human UGT1A1 activities in biological systems.J Pharm Anal. 2020 Jun;10(3):263-270. doi: 10.1016/j.jpha.2020.05.005. Epub 2020 May 23. J Pharm Anal. 2020. PMID: 32612873 Free PMC article.
-
Stereoselectivity of In Vivo Processes and Bioactivity of Farrerol Enantiomers.Molecules. 2025 May 3;30(9):2038. doi: 10.3390/molecules30092038. Molecules. 2025. PMID: 40363843 Free PMC article.
-
Development of a UHPLC-MS/MS method for the quantification of ilaprazole enantiomers in rat plasma and its pharmacokinetic application.J Pharm Anal. 2020 Dec;10(6):617-623. doi: 10.1016/j.jpha.2019.09.002. Epub 2019 Sep 17. J Pharm Anal. 2020. PMID: 33425456 Free PMC article.
-
Development and Validation of a Chiral Liquid Chromatographic Assay for Enantiomeric Separation and Quantification of Verapamil in Rat Plasma: Stereoselective Pharmacokinetic Application.Molecules. 2021 Apr 6;26(7):2091. doi: 10.3390/molecules26072091. Molecules. 2021. PMID: 33917412 Free PMC article.
-
Peptide and Protein Cysteine Modification Enabled by Hydrosulfuration of Ynamide.ACS Cent Sci. 2024 Aug 21;10(9):1742-1754. doi: 10.1021/acscentsci.4c01148. eCollection 2024 Sep 25. ACS Cent Sci. 2024. PMID: 39345815 Free PMC article.
References
-
- Rentsch K.M. The importance of stereoselective determination of drugs in the clinical laboratory. J. Biochem. Biophys. Methods. 2002;54:1–9. - PubMed
-
- Campo V.L., Bernardes L.S., Carvalho I. Stereoselectivity in drug metabolism: molecular mechanisms and analytical methods. Curr. Drug Metab. 2009;10:188–205. - PubMed
-
- Wang Y., Cao J., Wang X. Stereoselective transport and uptake of propranolol across human intestinal Caco-2 cell monolayers. Chirality. 2010;22:361–368. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

