LC-HRMS determination of piperine on rat dried blood spots: A pharmacokinetic study
- PMID: 29403958
- PMCID: PMC5762438
- DOI: 10.1016/j.jpha.2015.07.002
LC-HRMS determination of piperine on rat dried blood spots: A pharmacokinetic study
Abstract
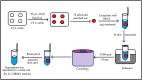
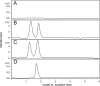
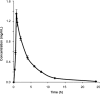
A liquid chromatography-high resolution mass spectrometry (LC-HRMS) method was developed and validated for the determination of piperine (PPR) on dried blood spots (DBS). DBS samples were prepared by spiking the whole blood with analyte to produce 30 µL of blood spots on specimen collection cards. Chromatographic separation was achieved on an Atlantis dC18 column using acetonitrile and water (0.1% formic acid) (85:15, v/v) as mobile phase in an isocratic mode of elution at a flow rate of 0.75 mL/min. MS detection was carried out in electrospray positive ion mode for the target ions and monitored at m/z 286.1465 for PPR and 272.1303 for the internal standard (IS). The developed method exhibited a linear dynamic range over 0.01-2000 ng/mL for PPR on DBS. The overall extraction recovery of PPR from DBS was 92.5%. Influence of hematocrit and spot volume on DBS was also evaluated and found to be well within the acceptable limits. The method was successfully applied to pharmacokinetic studies of PPR in rats.
Keywords: Dried blood spot; LC–HRMS; Pharmacokinetics; Piperine; Trichostachine.
Figures
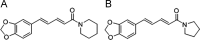

Similar articles
-
Quantitative determination of atenolol in dried blood spot samples by LC-HRMS: a potential method for assessing medication adherence.J Chromatogr B Analyt Technol Biomed Life Sci. 2012 May 15;897:72-9. doi: 10.1016/j.jchromb.2012.04.013. Epub 2012 Apr 16. J Chromatogr B Analyt Technol Biomed Life Sci. 2012. PMID: 22552005
-
Dexamethasone quantification in dried blood spot samples using LC-MS: The potential for application to neonatal pharmacokinetic studies.J Chromatogr B Analyt Technol Biomed Life Sci. 2010 Dec 1;878(31):3277-82. doi: 10.1016/j.jchromb.2010.10.009. Epub 2010 Oct 20. J Chromatogr B Analyt Technol Biomed Life Sci. 2010. PMID: 21071288
-
LC-MS/MS determination of pramipexole on rat dried blood spots: a pharmacokinetic study.J Chromatogr B Analyt Technol Biomed Life Sci. 2013 Aug 1;932:34-9. doi: 10.1016/j.jchromb.2013.06.012. Epub 2013 Jun 15. J Chromatogr B Analyt Technol Biomed Life Sci. 2013. PMID: 23831524
-
Simultaneous determination of bendamustine and γ-hydroxybendamustine in mice dried blood spots and its application in a mice pharmacokinetic study.J Pharm Biomed Anal. 2019 Sep 10;174:168-174. doi: 10.1016/j.jpba.2019.05.052. Epub 2019 May 28. J Pharm Biomed Anal. 2019. PMID: 31170630
-
The use of mass spectrometry to analyze dried blood spots.Mass Spectrom Rev. 2016 May-Jun;35(3):361-438. doi: 10.1002/mas.21441. Epub 2014 Sep 22. Mass Spectrom Rev. 2016. PMID: 25252132 Review.
Cited by
-
Molecular and pharmacological aspects of piperine as a potential molecule for disease prevention and management: evidence from clinical trials.Beni Suef Univ J Basic Appl Sci. 2022;11(1):16. doi: 10.1186/s43088-022-00196-1. Epub 2022 Jan 28. Beni Suef Univ J Basic Appl Sci. 2022. PMID: 35127957 Free PMC article. Review.
References
-
- Srinivasan K. Black pepper and its pungent principle-piperine: a review of diverse physiological effects. Crit. Rev. Food. Sci. Nutr. 2007;47:735–748. - PubMed
-
- Bae G.S., Kim M.S., Jung W.S. Inhibition of lipopolysaccharide-induced inflammatory responses by piperine. Eur. J. Pharmacol. 2010;642:154–162. - PubMed
-
- Lee S.A., Hong S.S., Han X.H. Piperine from the fruits of piper longum with inhibitory effect on monoamine oxidase and antidepressant-like activity. Chem. Pharm. Bull. 2005;53:832–835. - PubMed
-
- Li S., Wang C., Wang M. Antidepressant like effects of piperine in chronic mild stress treated mice and its possible mechanisms. Life Sci. 2007;80:1373–1381. - PubMed
-
- Atal C.K., Zutshi U., Rao P.G. Scientific evidence on the role of Ayurvedic herbals on bioavailability of drugs. J. Ethnopharmacol. 1981;4:229–232. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

