Dissolution method for delapril and manidipine combination tablets based on an absorption profile of manidipine
- PMID: 29403962
- PMCID: PMC5762453
- DOI: 10.1016/j.jpha.2015.10.002
Dissolution method for delapril and manidipine combination tablets based on an absorption profile of manidipine
Abstract
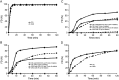
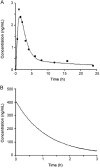
The present study describes the development and validation of a dissolution method for delapril (DEL) and manidipine (MAN) combination tablets, using a simulated absorption profile based on in vivo data for MAN. The suitable in vitro dissolution profile for this formulation was obtained using 900 mL of citrate buffer pH 3.2 at 37 °C±0.5 °C as dissolution medium and USP apparatus 2 (paddle) at 75 rpm. All samples were analyzed by a liquid chromatography (LC) method. Under these conditions, a significant linear relationship between the absorbed (calculated by deconvolution approach) and dissolved fractions of MAN was obtained (R=0.997) and an in vivo-in vitro (IVIV) correlation for this particular formulation containing MAN can be established. Validation parameters for dissolution methodology such as the specificity, linearity, accuracy and precision were also evaluated according to the international guidelines, giving results within the acceptable range. Therefore, the proposed dissolution conditions can be applied for the simultaneous release analysis of DEL and MAN from the solid dosage form, contributing to the improvement of the quality control of pharmaceutics and minimizing the number of bioavailability studies.
Keywords: Delapril; Dissolution; Manidipine; Validation; in vivo-in vitro correlation.
Figures



References
-
- Food and Drug Administration, Guidance for Industry: Dissolution Testing of Immediate Release Solid Oral Dosage Forms, Rockville, 1997a.
-
- Dressman J.B., Lennernas H. Marcel Dekker; New York: 2000. Oral Drug Absorption Prediction and Assessment.
-
- Food and Drug Administration, Guidance for Industry: Extended Release Oral Dosage Forms: Development, Evaluation, and Application of in vitro-in vivo Correlations, Rockville, 1997b. - PubMed
-
- Dressman J.B., Amidon G.L., Reppas C. Dissolution testing as a prognostic tool for oral drug absorption: immediate release dosage forms. Pharm. Res. 1998;15:11–22. - PubMed
-
- Onoyama K., Nanishi F., Okuda S. Pharmacokinetics of a new angiotensin I converting enzyme inhibitor (delapril) in patients with deteriorated kidney function and in normal control subjects. Clin. Pharmacol. Ther. 1988;43:242–249. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

