Determination of lercanidipine in human plasma by an improved UPLC-MS/MS method for a bioequivalence study
- PMID: 29403967
- PMCID: PMC5762449
- DOI: 10.1016/j.jpha.2015.09.001
Determination of lercanidipine in human plasma by an improved UPLC-MS/MS method for a bioequivalence study
Abstract
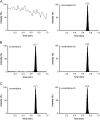
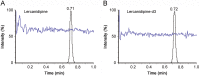
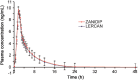
An improved and reliable ultra-performance liquid chromatography/tandem mass spectrometry (UPLC-MS/MS) method has been developed and validated for the determination of lercanidipine in human plasma. Plasma samples with lercanidipine-d3 as an internal standard (IS) were prepared by solid phase extraction on Phenomenex Strata-X cartridges using 100 µL of human plasma. Chromatographic analysis was performed on UPLC BEH C18 (50 mm×2.1 mm, 1.7 µm) column under isocratic conditions. Linear calibration curves were obtained over a wide dynamic concentration range of 0.010-20.0 ng/mL. Matrix effect was assessed by post-column infusion, post-extraction spiking and standard-line slope methods. The mean extraction recovery was >94% for the analyte and IS. Inter-batch and intra-batch precision (% CV) across five quality controls was <5.8%. Bioequivalence study was performed with 36 healthy subjects after oral administration of 10 mg of lercanidipine and the assay reproducibility was evaluated by reanalysis of 133 incurred samples.
Keywords: Bioequivalence; Human plasma; Lercanidipine; Solid phase extraction; UPLC–MS/MS.
Figures
References
-
- Hair P.I., Scott L.J., Perry C.M. Fixed-dose combination lercanidipine/enalapril. Drugs. 2007;67:95–106. - PubMed
-
- Fogari R., Mugellini A., Zoppi A. Differential effects of lercanidipine and nifedipine GITS on plasma norepinephrine in chronic treatment of hypertension. Am. J. Hypertens. 2003;16:596–599. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

