Study and ICH validation of a reverse-phase liquid chromatographic method for the quantification of the intact monoclonal antibody cetuximab
- PMID: 29403971
- PMCID: PMC5762446
- DOI: 10.1016/j.jpha.2015.11.007
Study and ICH validation of a reverse-phase liquid chromatographic method for the quantification of the intact monoclonal antibody cetuximab
Abstract
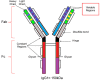
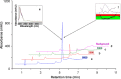
Cetuximab (CTX) is a potent chimeric mouse/human monoclonal antibody (mAb) approved worldwide for treatment of metastatic colorectal cancer. Among the various biological and physical analyses performed for full study on this biopharmaceutic, the determination of the concentration preparations throughout manufacturing and subsequent handling in hospital is particularly relevant. In the present work, the study and validation of a method for quantifying intact CTX by reverse-phase high-performance liquid chromatography with diode array detection ((RP)HPLC/DAD) is presented. With that end, we checked the performance of a chromatographic method for quantifying CTX and conducted a study to validate the method as stability-indicating in accordance with the International Conference on Harmonization guidelines (ICH) for biotechnological drugs; therefore, we evaluated linearity, accuracy, precision, detection and quantification limits, robustness and system suitability. The specificity of the method and the robustness of the mAb formulation against external stress factors were estimated by comprehensive chromatographic analysis by subjecting CTX to several informative stress conditions. As demonstrated, the method is rapid, accurate, and reproducible for CTX quantification. It was also successfully used to quantify CTX in a long-term stability study performed under hospital conditions.
Keywords: Biopharmaceuticals; Cetuximab; Diode detector; Reverse-phase high performance liquid chromatography; Stress study.
Figures



References
-
- Scientific discussion cetuximabmab EMEA, 2004. 〈http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_D...〉
-
- Dimitrov D.S. Therapeutic Proteins. In: Voynov V., Caravella J.A., editors. Vol. 2012. Springer; New York, New Jersey: 2012. pp. 1–26.
-
- Mould D.R., Sweeney K.R.D. The pharmacokinetics and pharmacodynamics of monoclonal antibodies. Curr. Opin. Drug. Discov. Dev. 2007;10:84–96. - PubMed
-
- Rosati S., Thompson N.J., Heck A.J.R. Tackling the increasing complexity of therapeutic monoclonal antibodies with mass spectrometry. TrAC-Trends Anal. Chem. 2013;48:72–80.
-
- Beck A., Wagner-Rousset E., Ayoub D. Characterization of therapeutic antibodies and related products. Anal. Chem. 2013;85:715–736. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

