Comparison of ESI- and APCI-LC-MS/MS methods: A case study of levonorgestrel in human plasma
- PMID: 29404004
- PMCID: PMC5762935
- DOI: 10.1016/j.jpha.2016.03.006
Comparison of ESI- and APCI-LC-MS/MS methods: A case study of levonorgestrel in human plasma
Abstract
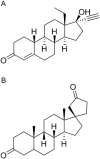
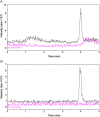
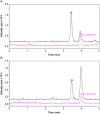
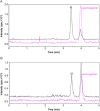
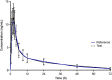
Electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI) techniques for liquid chromatography-tandem mass spectrometry (LC-MS/MS) determination of levonorgestrel were evaluated. In consideration of difference in ionization mechanism, the two ionization sources were compared in terms of LC conditions, MS parameters and performance of method. The sensitivity for detection of levonorgestrel with ESI was 0.25 ng/mL which was lower than 1 ng/mL with APCI. Matrix effects were evaluated for levonorgestrel and canrenone (internal standard, IS) in human plasma, and the results showed that APCI source appeared to be slightly less liable to matrix effect than ESI source. With an overall consideration, ESI was chosen as a better ionization technique for rapid and sensitive quantification of levonorgestrel. The optimized LC-ESI-MS/MS method was validated for a linear range of 0.25-50 ng/mL with a correlation coefficient ≥0.99. The intra- and inter-batch precision and accuracy were within 11.72% and 6.58%, respectively. The application of this method was demonstrated by a bioequivalence study following a single oral administration of 1.5 mg levonorgestrel tablets in 21 Chinese healthy female volunteers.
Keywords: Atmospheric pressure chemical ionization; Electrospray ionization; LC–MS/MS; Levonorgestrel; Pharmacokinetics.
Figures
Similar articles
-
Determination of urinary phytoestrogens by HPLC-MS/MS: a comparison of atmospheric pressure chemical ionization (APCI) and electrospray ionization (ESI).J Chromatogr B Analyt Technol Biomed Life Sci. 2008 Jan 1;861(1):145-50. doi: 10.1016/j.jchromb.2007.11.013. Epub 2007 Nov 21. J Chromatogr B Analyt Technol Biomed Life Sci. 2008. PMID: 18068558
-
Determination of levonorgestrel in human plasma by liquid chromatography-tandem mass spectrometry method: application to a bioequivalence study of two formulations in healthy volunteers.Biomed Chromatogr. 2008 May;22(5):519-26. doi: 10.1002/bmc.963. Biomed Chromatogr. 2008. PMID: 18254150
-
Monitoring phospholipids for assessment of ion enhancement and ion suppression in ESI and APCI LC/MS/MS for chlorpheniramine in human plasma and the importance of multiple source matrix effect evaluations.J Chromatogr B Analyt Technol Biomed Life Sci. 2008 Nov 15;875(2):333-43. doi: 10.1016/j.jchromb.2008.08.032. Epub 2008 Sep 18. J Chromatogr B Analyt Technol Biomed Life Sci. 2008. PMID: 18926779
-
Forced degradation and impurity profiling: recent trends in analytical perspectives.J Pharm Biomed Anal. 2013 Dec;86:11-35. doi: 10.1016/j.jpba.2013.07.013. Epub 2013 Jul 31. J Pharm Biomed Anal. 2013. PMID: 23969330 Review.
-
Response in Ambient Low Temperature Plasma Ionization Compared to Electrospray and Atmospheric Pressure Chemical Ionization for Mass Spectrometry.Int J Anal Chem. 2018 Dec 18;2018:5647536. doi: 10.1155/2018/5647536. eCollection 2018. Int J Anal Chem. 2018. PMID: 30723503 Free PMC article. Review.
Cited by
-
Comparison of electrospray and UniSpray, a novel atmospheric pressure ionization interface, for LC-MS/MS analysis of 81 pesticide residues in food and water matrices.Anal Bioanal Chem. 2019 Aug;411(20):5099-5113. doi: 10.1007/s00216-019-01886-z. Epub 2019 May 31. Anal Bioanal Chem. 2019. PMID: 31152225 Free PMC article.
-
Atmospheric pressure chemical ionisation mass spectrometry for the routine analysis of low molecular weight analytes.Eur J Mass Spectrom (Chichester). 2021 Feb;27(1):13-28. doi: 10.1177/14690667211005055. Epub 2021 Apr 5. Eur J Mass Spectrom (Chichester). 2021. PMID: 33820464 Free PMC article.
-
Simultaneous Determination for Nine Kinds of N-Nitrosamines Compounds in Groundwater by Ultra-High-Performance Liquid Chromatography Coupled with Triple Quadrupole Mass Spectrometry.Int J Environ Res Public Health. 2022 Dec 12;19(24):16680. doi: 10.3390/ijerph192416680. Int J Environ Res Public Health. 2022. PMID: 36554561 Free PMC article.
-
Development and validation of a sensitive method to quantify a poorly ionized drug, pocapavir, in C57BL/6 mouse plasma.Bioanalysis. 2025 Mar;17(6):393-398. doi: 10.1080/17576180.2025.2480533. Epub 2025 Mar 21. Bioanalysis. 2025. PMID: 40118813
References
-
- Kook K., Gabelnick H., Duncan G. Pharmacokinetics of levonorgestrel 0.75 mg tablets. Contraception. 2002;66:73–76. - PubMed
-
- Ding W.X., Qi X.R., Fu Q. Pharmacokinetics and pharmacodynamics of sterylglucoside-modified liposomes for levonorgestrel delivery via nasal route. Drug Deliv. 2007;14:101–104. - PubMed
-
- Sitruk-Ware R., Nath A. Characteristics and metabolic effects of estrogen and progestins contained in oral contraceptive pills. Best Pract. Res. Clin. Endocrinol. Metab. 2013;27:13–24. - PubMed
-
- Ananthula S., Janagam D.R., Jamalapuram S. Development and validation of sensitive LC/MS/MS method for quantitative bioanalysis of levonorgestrel in rat plasma and application to pharmacokinetics study. J. Chromatogr. B. 2015;1003:47–53. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

