Development of an HPLC-UV assay method for the simultaneous quantification of nine antiretroviral agents in the plasma of HIV-infected patients
- PMID: 29404009
- PMCID: PMC5762929
- DOI: 10.1016/j.jpha.2016.05.008
Development of an HPLC-UV assay method for the simultaneous quantification of nine antiretroviral agents in the plasma of HIV-infected patients
Abstract
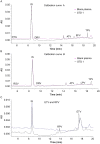
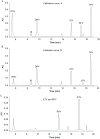
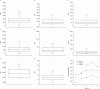
A new method using high-performance liquid chromatography coupled with ultra violet detection (HPLC-UV) was developed and validated for the simultaneous quantification of atazanavir, dolutegravir, darunavir, efavirenz, etravirine lopinavir, raltegravir, rilpivirine and tipranavir in human plasma. For the first time we reported here the development and validation of an HPLC-UV assay to quantify the frequently administered 9 antiretroviral compounds including dolutegravir and rilpivirine. A simple solid phase extraction procedure was applied to 500 µL aliquots of plasma. The chromatographic separation of the drugs and internal standard (quinoxaline) was achieved with a gradient of acetonitrile and sodium acetate buffer on a C18 reverse-phase analytical column with a 25 min analytical run time. Calibration curves were optimised according to the therapeutic range of drug concentrations in patients, and the coefficient of determination (r2) was higher than 0.99 for all analytes. Mean intraday and interday precisions (RSD) for all compounds were less than 15.0%, and the mean accuracy (% deviation from nominal concentration) was also found to be less than 15.0%. Extraction recovery range was between 80% and 120% for all drugs analysed. The solid phase extraction and HPLC-UV method enable a specific, sensitive, and reliable simultaneous determination of nine antiretroviral agents in plasma. Good extraction efficiency and low limit of HPLC-UV quantification make this method suitable for use in clinical trials and therapeutic drug monitoring.
Keywords: Antiretrovirals; Bioanalytical method validation; HPLC–UV.
Figures
Similar articles
-
An HPLC-PDA method for the simultaneous quantification of the HIV integrase inhibitor raltegravir, the new nonnucleoside reverse transcriptase inhibitor etravirine, and 11 other antiretroviral agents in the plasma of HIV-infected patients.Ther Drug Monit. 2008 Dec;30(6):662-9. doi: 10.1097/FTD.0b013e318189596d. Ther Drug Monit. 2008. PMID: 18824956
-
HPLC-MS method for the simultaneous quantification of the new HIV protease inhibitor darunavir, and 11 other antiretroviral agents in plasma of HIV-infected patients.J Chromatogr B Analyt Technol Biomed Life Sci. 2007 Nov 15;859(2):234-40. doi: 10.1016/j.jchromb.2007.10.003. Epub 2007 Oct 7. J Chromatogr B Analyt Technol Biomed Life Sci. 2007. PMID: 17964231
-
Development and Validation of a Chromatographic Ultraviolet Method for the Simultaneous Quantification of Dolutegravir and Rilpivirine in Human Plasma.Ther Drug Monit. 2016 Jun;38(3):407-13. doi: 10.1097/FTD.0000000000000290. Ther Drug Monit. 2016. PMID: 26885814
-
Development and validation of an HPLC-UV method for quantification of elvitegravir and two other new antiretrovirals, dolutegravir and rilpivirine, in the plasma of HIV-positive patients.Biomed Chromatogr. 2018 May 4:e4274. doi: 10.1002/bmc.4274. Online ahead of print. Biomed Chromatogr. 2018. PMID: 29726595
-
Validation of an electrospray ionization LC-MS/MS method for quantitative analysis of raltegravir, etravirine, and 9 other antiretroviral agents in human plasma samples.Ther Drug Monit. 2009 Dec;31(6):695-702. doi: 10.1097/FTD.0b013e3181c05adf. Ther Drug Monit. 2009. PMID: 19865000
Cited by
-
Assessment of lamivudine, zidovudine, lopinavir, and ritonavir plasma levels in HIV-positive pregnant women: Drug monitoring application to improve patient safety.Medicine (Baltimore). 2020 May 29;99(22):e20487. doi: 10.1097/MD.0000000000020487. Medicine (Baltimore). 2020. PMID: 32481459 Free PMC article.
-
Therapeutic Drug Monitoring of Long-Acting Rilpivirine and Cabotegravir for Treatment of HIV-1 Infection-A Case Series of Five Patients With One Virologic Failure After Development of Two-Class Resistance.Open Forum Infect Dis. 2024 Aug 23;11(9):ofae480. doi: 10.1093/ofid/ofae480. eCollection 2024 Sep. Open Forum Infect Dis. 2024. PMID: 39286033 Free PMC article.
-
Antiretroviral concentration measurements as an additional tool to manage virologic failure in resource limited settings: a case control study.AIDS Res Ther. 2019 Dec 6;16(1):39. doi: 10.1186/s12981-019-0255-x. AIDS Res Ther. 2019. PMID: 31810468 Free PMC article.
-
Detection and quantification of Covid-19 antiviral drugs in biological fluids and tissues.Talanta. 2021 Mar 1;224:121862. doi: 10.1016/j.talanta.2020.121862. Epub 2020 Nov 5. Talanta. 2021. PMID: 33379073 Free PMC article. Review.
-
Intranasal Administration of Dolutegravir-Loaded Nanoemulsion-Based In Situ Gel for Enhanced Bioavailability and Direct Brain Targeting.Gels. 2023 Feb 3;9(2):130. doi: 10.3390/gels9020130. Gels. 2023. PMID: 36826300 Free PMC article.
References
-
- Sackoff J.E., Hanna D.B., Pfeiffer M.R. Causes of death among persons with aids in the era of highly active antiretroviral therapy: New York City. Ann. Intern. Med. 2006;145:397–406. - PubMed
-
- Shah S.S., McGowan J.P., Smith C. Comorbid conditions, treatment, and health maintenance in older persons with human immunodeficiency virus infection in New York City. Clin. Infect. Dis. 2002;35:1238–1243. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

