Binding interaction of phosphorus heterocycles with bovine serum albumin: A biochemical study
- PMID: 29404014
- PMCID: PMC5686865
- DOI: 10.1016/j.jpha.2016.05.009
Binding interaction of phosphorus heterocycles with bovine serum albumin: A biochemical study
Abstract
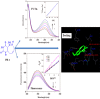
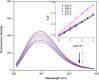
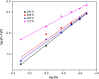
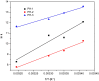
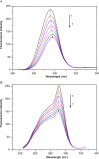
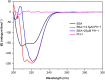
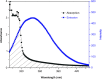
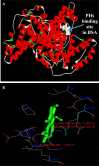
Interaction between bovine serum albumin (BSA) and phosphorus heterocycles (PHs) was studied using multi-spectroscopic techniques. The results indicated the high binding affinity of PHs to BSA as it quenches the intrinsic fluorescence of BSA. The experimental data suggested the fluorescence quenching mechanism between PHs and BSA as a dynamic quenching. From the UV-vis studies, the apparent association constant (Kapp) was found to be 9.25×102, 1.27×104 and 9.01×102 L/mol for the interaction of BSA with PH-1, PH-2 and PH-3 respectively. According to the Förster's non-radiation energy transfer (FRET) theory, the binding distances between BSA and PHs were calculated. The binding distances (r) of PH-1, PH-2 and PH-3 were found to be 2.86, 3.03, and 5.12 nm, respectively, indicating energy transfer occurs between BSA and PHs. The binding constants of the PHs obtained from the fluorescence quenching data were found to be decreased with increase of temperature. The negative values of the thermodynamic parameters ΔH, ΔS and ΔG at different temperatures revealed that the binding process is spontaneous; hydrogen bonds and van der Waals interaction were the main force to stabilize the complex. The microenvironment of the protein-binding site was studied by synchronous fluorescence and circular dichroism (CD) techniques and data indicated that the conformation of BSA changed in the presence of PHs. Finally, we studied the BSA-PHs docking using Autodock and results suggest that PHs is located in the cleft between the domains of BSA.
Keywords: BSA; BSA-PHs docking; Phosphorus heterocycles; Spectroscopy.
Figures

References
-
- Carter D.C., Ho J.X. Structure of serum albumin. Adv. Protein Chem. 1994;45:153–203. - PubMed
-
- Mathias U., Jung M. Determination of drug-serum protein interactions via fluorescence polarization measurements. Anal. Bioanal. Chem. 2007;388:1147–1156. - PubMed
-
- Bourasssa P., Kanakis C.D., Tarantilis P. Resveratrol, genistein, and curcumin bind bovine serum albumin. J. Phys. Chem. B. 2010;114:3348–3354. - PubMed
-
- Smith A.B., Taylor C.M., Benkovic S.J. Peptide bond formation via catalytic antibodies: synthesis of a novel phosphonate diester hapten. Tetrahedron Lett. 1994;35:6853–6856.
-
- Morita I., Kunimoto K., Tsuda M. Synthesis and antihypertensive activities of 1,4-dihydropyridine-5-phosphonate derivatives. Chem. Pharm. Bull. 1987;35:4144–4154. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

