Fabrication of an electrochemical sensor for determination of doxorubicin in human plasma and its interaction with DNA
- PMID: 29404015
- PMCID: PMC5686857
- DOI: 10.1016/j.jpha.2016.07.005
Fabrication of an electrochemical sensor for determination of doxorubicin in human plasma and its interaction with DNA
Abstract
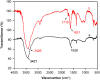
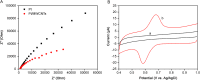
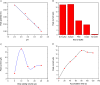
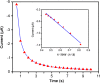
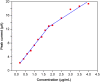
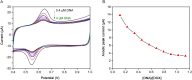
In this work, an electrochemical sensor was fabricated for determination of an anthracycline, doxorubicin (DOX) as a chemotherapy drug in plasma based on multi-walled carbon nanotubes modified platinum electrode (Pt/MWCNTs). DOX was effectively accumulated on the surface of modified electrode and generated a pair of redox peaks at around 0.522 and 0.647 V (vs. Ag/AgCl) in Britton Robinson (B-R) buffer (pH 4.0, 0.1 M). The electrochemical parameters including pH, type of buffer, accumulation time, amount of modifier and scan rate were optimized. Under the optimized conditions, there was a linear correlation between cathodic peak current and concentration of DOX in the range of 0.05-4.0 µg/mL with the detection limit of 0.002 µg/mL. The number of electron transfers (n) and electron transfer-coefficient (α) were estimated as 2.0 and 0.25, respectively. The constructed sensor displayed excellent precision, sensitivity, repeatability and selectivity in the determination of doxorubicin in plasma. Moreover, cyclic voltammetry studies of DOX in the presence of DNA showed an intercalation mechanism with binding constant (Kb) of 1.12×105 L/mol.
Keywords: Doxorubicin; Doxorubicin-DNA interaction; Electrochemical sensor; Human plasma; MWCNTs.
Figures

References
-
- Padigi S.K., Reddy R.K.K., Prasad S. Carbon nanotube based aliphatic hydrocarbon sensor. Biosens. Bioelectron. 2007;22:829–837. - PubMed
-
- Baughman R.H., Zakhidov A.A., de Heer W.A. Carbon nanotubes – the route toward applications. Science. 2002;297:787–792. - PubMed
-
- Rao C.N., Satishkumar B.C., Govindaraj A. Nanotubes. Chemphyschem. 2001;2:78–105. - PubMed
-
- Ajayan P. Nanotubes from carbon. Chem. Rev. 1999;99:1787–1800. - PubMed
-
- Goyal R.N., Gupta V.K., Chatterjee S. Voltammetric biosensors for the determination of paracetamol at carbon nanotube modified pyrolytic graphite electrode. Sens. Actuators B-Chem. 2010;149:252–258.
LinkOut - more resources
Full Text Sources
Other Literature Sources

