Multi-spectroscopic characterization of bovine serum albumin upon interaction with atomoxetine
- PMID: 29404031
- PMCID: PMC5790691
- DOI: 10.1016/j.jpha.2016.10.001
Multi-spectroscopic characterization of bovine serum albumin upon interaction with atomoxetine
Abstract
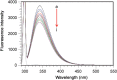
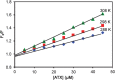
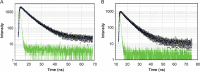
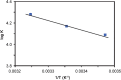
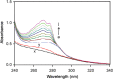
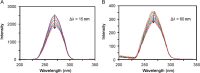
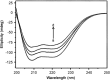
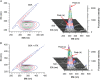
The quenching interaction of atomoxetine (ATX) with bovine serum albumin (BSA) was studied in vitro under optimal physiological condition (pH=7.4) by multi-spectroscopic techniques. The mechanism of ATX-BSA system was a dynamic quenching process and was confirmed by the fluorescence spectra and lifetime measurements. The number of binding sites, binding constants and other binding characteristics were computed. Thermodynamic parameters ∆H° and ∆S° indicated that intermolecular hydrophobic forces predominantly stabilized the drug-protein system. The average binding distance between BSA and ATX was studied by Försters theory. UV-absorption, Fourier transform infrared spectroscopy (FT-IR), circular dichroism (CD), synchronous spectra and three-dimensional (3D) fluorescence spectral results revealed the changes in micro-environment of secondary structure of protein upon the interaction with ATX. Displacement of site probes and the effects of some common metal ions on the binding of ATX with BSA interaction were also studied.
Keywords: 3D fluorescence spectra; Atomoxetine; Bovine serum albumin; Energy transfer; FT-IR; Lifetime measurement.
Figures




References
-
- Yu J., Li B., Dai P., Ge S. Molecular simulation of the interaction between novel type rhodanine derivative probe and bovine serum albumin. Spectrochim. Acta A. 2009;74:277–281. - PubMed
-
- Hu Y.J., Liu Y., Shen X.S. Studies on the interaction between 1-hexylcarbamoyl-5-fluorouracil and bovine serum albumin. J. Mol. Struct. 2005;738:143–147.
-
- Sandhya B., Hegde A.H., Kalanur S.S. Interaction of triprolidine hydrochloride with serum albumins: thermodynamic and binding characteristics, and influence of site probes. J. Pharm. Biomed. Anal. 2011;54:1180–1186. - PubMed
-
- Zhao H.W., Ge M., Zhang Z.X. Spectroscopic studies on the interaction between riboflavin and albumins. Spectrochim. Acta A. 2006;65:811–817. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

