The emergence of NS5B resistance associated substitution S282T after sofosbuvir-based treatment
- PMID: 29404477
- PMCID: PMC5678900
- DOI: 10.1002/hep4.1060
The emergence of NS5B resistance associated substitution S282T after sofosbuvir-based treatment
Abstract
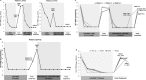
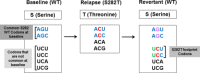
S282T in NS5B is the primary amino acid substitution associated with resistance to sofosbuvir (SOF) but has rarely been detected in patients treated with a SOF-based regimen. Here, the emergence and fitness of the S282T substitution in virologic failure patients administered SOF-based regimens across the SOF and ledipasvir (LDV)/SOF phase 2 and 3 programs was evaluated. Plasma samples collected at baseline and at virologic failure were amplified and deep sequenced (1% cutoff). To date, over 12,000 patients have been treated in SOF or LDV/SOF phase 2 and 3 studies. Of these, deep sequencing was available at baseline in 8598 patients (62.4% genotype [GT] 1, 10.7% GT2, 20.9% GT3, and 6.0% GT4-6) and at virologic failure in 901 patients. In the 8598 patients, no S282T substitution was detected at baseline; at virologic failure, 10 of the 901 (1%) patients had S282T detected. The SOF-based regimen associated with treatment-emergent S282T was SOF monotherapy in two patients, retreatment with LDV/SOF in prior LDV/SOF failures in three patients, LDV/SOF for 8 weeks in 1 GT1 patient, LDV/SOF for 12 weeks in 1 patient each with GT3, GT4, and GT5, and LDV/SOF + ribavirin for 12 weeks in 1 GT6 patient. Nine of 10 patients with emergent S282T received an SOF-based retreatment regimen, eight of whom achieved sustained virologic response 12 weeks after treatment and one of whom failed retreatment. Conclusion: The emergence of S282T substitution was rare in patients who fail SOF-based regimens. Successful retreatment of prior SOF failure patients is possible in the presence of S282T substitution with SOF in combination with various direct-acting antiviral agents. (Hepatology Communications 2017;1:538-549).
Figures

References
-
- Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis 2005;5:558‐567. - PubMed
-
- Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 2014;370:1889‐1898. - PubMed
-
- Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med 2014;370:1483‐1493. - PubMed
-
- Gane EJ HR, Yang Y, Svarovskaia E, Pang PS, McHutchison JG, Stedman CA. ISVHLD: ledipasvir/sofosbuvir single tablet regimen is effective in patients with HCV genotype 2 infection [Abstract]. Presented at the 15th International Symposium on Viral Hepatitis and Liver Diseases (ISVHLD); Berlin, Germany; June 26‐28, 2015. Abstract O-25.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

