Triage by cervical length sonographic measurements for targeted therapy in threatened preterm labor: A double blind randomized clinical trial
- PMID: 29404531
- PMCID: PMC5780555
Triage by cervical length sonographic measurements for targeted therapy in threatened preterm labor: A double blind randomized clinical trial
Abstract
Background: Preterm labor and birth are associated with several neonatal complications including respiratory distress syndrome and intraventricular hemorrhage. Differentiating true and false labor pain is a dilemma to obstetricians.
Objective: To elucidate the role of cervical length measurement in prediction of birth in pregnant women with threatened preterm labor.
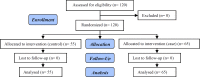
Materials and methods: In this double blind randomized clinical trial, 120 women with gestational age <34 wk who presented painful uterine contractions randomly assigned to undergo measurement of cervical length. Patients were registered in the hospital and a unit number was given. Based on the unit numbers, patients were randomly assigned to two groups using a computerized random digit generator. All participants were managed accordingly (n=65) or to receive tocolysis as planned (n=55). Tocolysis was prescribed when cervical length was <15 mm while those with cervical length ≥15 mm were managed expectantly. Delivery within 7 days of the presentation was the primary outcome.
Results: This RCT showed in case group, 78.9% of patient with cervical length <15 mm were delivered within 7 days and only 21.1% of them maintained their pregnancy. Of those with cervical length ≥15 mm, only 15.2% were delivered within the study period and the rest (84.8%) maintained their pregnancy (p<0.001).
Conclusion: "Our results indicate that in women who presented preterm labor symptoms, cervical length measurement will result in decreased unnecessary tocolytic treatment. Women with cervical length ≥15mm should not receive tocolysis, however, withholding corticosteroid therapy in these patients needs further evidence.
Keywords: Cervical length; Preterm labor; Tocolysis; Ultrasonography..
Conflict of interest statement
The authors declare that there is no conflict of interests regarding the publication of this paper.
Figures
References
-
- Martin JA. Centers for Disease Control and Prevention (CDC) Preterm births-united states,2007. MMWR. 2011;60 ((Suppl.)):78–79. - PubMed
-
- Min J, Watson HA, Hezelgrave NL, Seed PT, Shennan A. Ability of preterm surveillance clinic triage risk of preterm birth: a prospective cohortstudy. Ultrasound Obstet Gynecol. 2016;48:38–42. - PubMed
-
- Creasy RK. Preterm birth prevention: where are we? Am J Obstet Gynecol. 1993;168:1223–1230. - PubMed
-
- King J, Flenady V. Prophylactic antibiotics for inhibiting preterm labor with intact membranes. Cochrane Database Syst Rev. 2002;4:CD000246. - PubMed
LinkOut - more resources
Full Text Sources