Efficacy and Safety of Secukinumab in Elderly Subjects with Moderate to Severe Plaque Psoriasis: A Pooled Analysis of Phase III Studies
- PMID: 29404966
- PMCID: PMC5847154
- DOI: 10.1007/s40266-018-0520-z
Efficacy and Safety of Secukinumab in Elderly Subjects with Moderate to Severe Plaque Psoriasis: A Pooled Analysis of Phase III Studies
Abstract
Background: Psoriasis is a chronic inflammatory skin disease, affecting patients of a wide age range, including elderly patients. Elderly patients can respond differently to drug treatments and can be more vulnerable to adverse reactions. There are limited data on biologic therapies for psoriasis in elderly subjects. Secukinumab, a fully human monoclonal antibody that selectively neutralizes IL-17A, has proven significant efficacy in the treatment of moderate to severe psoriasis.
Aims: A post-hoc analysis of three phase III trials (ERASURE, FIXTURE and CLEAR) was performed to evaluate the efficacy and safety of secukinumab in elderly subjects.
Methods: Studies were multicentre, randomized, parallel-group, double-blind, 52-week phase III trials in subjects with moderate to severe plaque psoriasis. For efficacy analyses, 67 elderly subjects (≥ 65 years) treated with secukinumab 300 mg were compared with 841 younger subjects (18-64 years). Psoriasis Area and Severity Index (PASI), Dermatological Life Quality Index (DLQI) and safety were analysed.
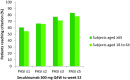
Results: Elderly subjects had higher baseline frequencies of cardiovascular and metabolic disorders. Secukinumab efficacy in elderly subjects was comparable to that in younger subjects throughout 52 weeks of treatment. PASI 75 response was reached by 81.8% of elderly subjects and 79.4% of younger subjects at Week 52. Similar rates of DLQI 0/1 response were observed. The total rate of adverse events was similar between elderly and younger subjects.
Conclusions: Secukinumab at the recommended dose (300 mg) is effective and acceptably safe in subjects aged ≥ 65 years with moderate to severe psoriasis, with quality-of-life benefits, despite an increased prevalence of cardiovascular and metabolic comorbidities in this population.
Conflict of interest statement
Funding
The study was funded by Novartis Pharma GmbH, Germany.
Conflict of interest
AK has received honoraria from Novartis, Lilly, Leo Pharma, Almirall, Janssen, UCB, MSD and Pfizer and has received fees for board participation from Novartis, Leo Pharma, Janssen and Lilly. CP is an employee of Novartis Pharma AG. VB is an employee of Novartis Healthcare Pvt. Ltd. MR is an employee of Novartis Pharma GmbH.
Figures


Similar articles
-
Secukinumab long-term safety experience: A pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis.J Am Acad Dermatol. 2016 Jul;75(1):83-98.e4. doi: 10.1016/j.jaad.2016.03.024. Epub 2016 May 12. J Am Acad Dermatol. 2016. PMID: 27180926
-
Secukinumab in plaque psoriasis--results of two phase 3 trials.N Engl J Med. 2014 Jul 24;371(4):326-38. doi: 10.1056/NEJMoa1314258. Epub 2014 Jul 9. N Engl J Med. 2014. PMID: 25007392 Clinical Trial.
-
Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial.J Am Acad Dermatol. 2015 Sep;73(3):400-9. doi: 10.1016/j.jaad.2015.05.013. Epub 2015 Jun 17. J Am Acad Dermatol. 2015. PMID: 26092291 Clinical Trial.
-
Secukinumab (AIN457) for the treatment of psoriasis.Expert Rev Clin Immunol. 2015;11(11):1177-88. doi: 10.1586/1744666X.2015.1095092. Epub 2015 Oct 1. Expert Rev Clin Immunol. 2015. PMID: 26428036 Review.
-
Safety of secukinumab in the treatment of psoriasis.Expert Opin Drug Saf. 2016 Oct;15(10):1413-20. doi: 10.1080/14740338.2016.1221923. Epub 2016 Aug 22. Expert Opin Drug Saf. 2016. PMID: 27545070 Review.
Cited by
-
Efficacy and safety of biological agents for the treatment of pediatric patients with psoriasis: A bayesian analysis of six high-quality randomized controlled trials.Front Immunol. 2022 Aug 19;13:896550. doi: 10.3389/fimmu.2022.896550. eCollection 2022. Front Immunol. 2022. PMID: 36081503 Free PMC article.
-
Effectiveness of Ixekizumab in Elderly Patients for the Treatment of Moderate-to-Severe Psoriasis: Results From a Multicenter, Retrospective Real-Life Study in the Lazio Region.Dermatol Pract Concept. 2024 Jul 1;14(3):e2024166. doi: 10.5826/dpc.1403a166. Dermatol Pract Concept. 2024. PMID: 39122514 Free PMC article.
-
Drug Survival, Safety, and Effectiveness of Biologics in Older Patients with Psoriasis: A Comparison with Younger Patients-A BioCAPTURE Registry Study.Drugs Aging. 2022 Sep;39(9):715-727. doi: 10.1007/s40266-022-00961-y. Epub 2022 Jul 21. Drugs Aging. 2022. PMID: 35859228 Free PMC article.
-
Psoriasis and Metabolic Syndrome: Comorbidities and Environmental and Therapeutic Implications.Cureus. 2019 Dec 12;11(12):e6369. doi: 10.7759/cureus.6369. Cureus. 2019. PMID: 31938651 Free PMC article. Review.
-
Adverse events associated with anti-IL-17 agents for psoriasis and psoriatic arthritis: a systematic scoping review.Front Immunol. 2023 Jan 31;14:993057. doi: 10.3389/fimmu.2023.993057. eCollection 2023. Front Immunol. 2023. PMID: 36817423 Free PMC article.
References
-
- Gan EY, Chong WS, Tey HL. Therapeutic strategies in psoriasis patients with psoriatic arthritis: focus on new agents. BioDrugs Clin Immunother Biopharm. Gene Ther. 2013;27(4):359–373. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

