Efficacy and Safety of Secukinumab in Elderly Subjects with Moderate to Severe Plaque Psoriasis: A Pooled Analysis of Phase III Studies
- PMID: 29404966
- PMCID: PMC5847154
- DOI: 10.1007/s40266-018-0520-z
Efficacy and Safety of Secukinumab in Elderly Subjects with Moderate to Severe Plaque Psoriasis: A Pooled Analysis of Phase III Studies
Abstract
Background: Psoriasis is a chronic inflammatory skin disease, affecting patients of a wide age range, including elderly patients. Elderly patients can respond differently to drug treatments and can be more vulnerable to adverse reactions. There are limited data on biologic therapies for psoriasis in elderly subjects. Secukinumab, a fully human monoclonal antibody that selectively neutralizes IL-17A, has proven significant efficacy in the treatment of moderate to severe psoriasis.
Aims: A post-hoc analysis of three phase III trials (ERASURE, FIXTURE and CLEAR) was performed to evaluate the efficacy and safety of secukinumab in elderly subjects.
Methods: Studies were multicentre, randomized, parallel-group, double-blind, 52-week phase III trials in subjects with moderate to severe plaque psoriasis. For efficacy analyses, 67 elderly subjects (≥ 65 years) treated with secukinumab 300 mg were compared with 841 younger subjects (18-64 years). Psoriasis Area and Severity Index (PASI), Dermatological Life Quality Index (DLQI) and safety were analysed.
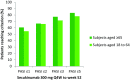
Results: Elderly subjects had higher baseline frequencies of cardiovascular and metabolic disorders. Secukinumab efficacy in elderly subjects was comparable to that in younger subjects throughout 52 weeks of treatment. PASI 75 response was reached by 81.8% of elderly subjects and 79.4% of younger subjects at Week 52. Similar rates of DLQI 0/1 response were observed. The total rate of adverse events was similar between elderly and younger subjects.
Conclusions: Secukinumab at the recommended dose (300 mg) is effective and acceptably safe in subjects aged ≥ 65 years with moderate to severe psoriasis, with quality-of-life benefits, despite an increased prevalence of cardiovascular and metabolic comorbidities in this population.
Conflict of interest statement
Funding
The study was funded by Novartis Pharma GmbH, Germany.
Conflict of interest
AK has received honoraria from Novartis, Lilly, Leo Pharma, Almirall, Janssen, UCB, MSD and Pfizer and has received fees for board participation from Novartis, Leo Pharma, Janssen and Lilly. CP is an employee of Novartis Pharma AG. VB is an employee of Novartis Healthcare Pvt. Ltd. MR is an employee of Novartis Pharma GmbH.
Figures


References
-
- Gan EY, Chong WS, Tey HL. Therapeutic strategies in psoriasis patients with psoriatic arthritis: focus on new agents. BioDrugs Clin Immunother Biopharm. Gene Ther. 2013;27(4):359–373. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

