Systemic treatments for metastatic cutaneous melanoma
- PMID: 29405038
- PMCID: PMC6491081
- DOI: 10.1002/14651858.CD011123.pub2
Systemic treatments for metastatic cutaneous melanoma
Abstract
Background: The prognosis of people with metastatic cutaneous melanoma, a skin cancer, is generally poor. Recently, new classes of drugs (e.g. immune checkpoint inhibitors and small-molecule targeted drugs) have significantly improved patient prognosis, which has drastically changed the landscape of melanoma therapeutic management. This is an update of a Cochrane Review published in 2000.
Objectives: To assess the beneficial and harmful effects of systemic treatments for metastatic cutaneous melanoma.
Search methods: We searched the following databases up to October 2017: the Cochrane Skin Group Specialised Register, CENTRAL, MEDLINE, Embase and LILACS. We also searched five trials registers and the ASCO database in February 2017, and checked the reference lists of included studies for further references to relevant randomised controlled trials (RCTs).
Selection criteria: We considered RCTs of systemic therapies for people with unresectable lymph node metastasis and distant metastatic cutaneous melanoma compared to any other treatment. We checked the reference lists of selected articles to identify further references to relevant trials.
Data collection and analysis: Two review authors extracted data, and a third review author independently verified extracted data. We implemented a network meta-analysis approach to make indirect comparisons and rank treatments according to their effectiveness (as measured by the impact on survival) and harm (as measured by occurrence of high-grade toxicity). The same two review authors independently assessed the risk of bias of eligible studies according to Cochrane standards and assessed evidence quality based on the GRADE criteria.
Main results: We included 122 RCTs (28,561 participants). Of these, 83 RCTs, encompassing 21 different comparisons, were included in meta-analyses. Included participants were men and women with a mean age of 57.5 years who were recruited from hospital settings. Twenty-nine studies included people whose cancer had spread to their brains. Interventions were categorised into five groups: conventional chemotherapy (including single agent and polychemotherapy), biochemotherapy (combining chemotherapy with cytokines such as interleukin-2 and interferon-alpha), immune checkpoint inhibitors (such as anti-CTLA4 and anti-PD1 monoclonal antibodies), small-molecule targeted drugs used for melanomas with specific gene changes (such as BRAF inhibitors and MEK inhibitors), and other agents (such as anti-angiogenic drugs). Most interventions were compared with chemotherapy. In many cases, trials were sponsored by pharmaceutical companies producing the tested drug: this was especially true for new classes of drugs, such as immune checkpoint inhibitors and small-molecule targeted drugs.When compared to single agent chemotherapy, the combination of multiple chemotherapeutic agents (polychemotherapy) did not translate into significantly better survival (overall survival: HR 0.99, 95% CI 0.85 to 1.16, 6 studies, 594 participants; high-quality evidence; progression-free survival: HR 1.07, 95% CI 0.91 to 1.25, 5 studies, 398 participants; high-quality evidence. Those who received combined treatment are probably burdened by higher toxicity rates (RR 1.97, 95% CI 1.44 to 2.71, 3 studies, 390 participants; moderate-quality evidence). (We defined toxicity as the occurrence of grade 3 (G3) or higher adverse events according to the World Health Organization scale.)Compared to chemotherapy, biochemotherapy (chemotherapy combined with both interferon-alpha and interleukin-2) improved progression-free survival (HR 0.90, 95% CI 0.83 to 0.99, 6 studies, 964 participants; high-quality evidence), but did not significantly improve overall survival (HR 0.94, 95% CI 0.84 to 1.06, 7 studies, 1317 participants; high-quality evidence). Biochemotherapy had higher toxicity rates (RR 1.35, 95% CI 1.14 to 1.61, 2 studies, 631 participants; high-quality evidence).With regard to immune checkpoint inhibitors, anti-CTLA4 monoclonal antibodies plus chemotherapy probably increased the chance of progression-free survival compared to chemotherapy alone (HR 0.76, 95% CI 0.63 to 0.92, 1 study, 502 participants; moderate-quality evidence), but may not significantly improve overall survival (HR 0.81, 95% CI 0.65 to 1.01, 2 studies, 1157 participants; low-quality evidence). Compared to chemotherapy alone, anti-CTLA4 monoclonal antibodies is likely to be associated with higher toxicity rates (RR 1.69, 95% CI 1.19 to 2.42, 2 studies, 1142 participants; moderate-quality evidence).Compared to chemotherapy, anti-PD1 monoclonal antibodies (immune checkpoint inhibitors) improved overall survival (HR 0.42, 95% CI 0.37 to 0.48, 1 study, 418 participants; high-quality evidence) and probably improved progression-free survival (HR 0.49, 95% CI 0.39 to 0.61, 2 studies, 957 participants; moderate-quality evidence). Anti-PD1 monoclonal antibodies may also result in less toxicity than chemotherapy (RR 0.55, 95% CI 0.31 to 0.97, 3 studies, 1360 participants; low-quality evidence).Anti-PD1 monoclonal antibodies performed better than anti-CTLA4 monoclonal antibodies in terms of overall survival (HR 0.63, 95% CI 0.60 to 0.66, 1 study, 764 participants; high-quality evidence) and progression-free survival (HR 0.54, 95% CI 0.50 to 0.60, 2 studies, 1465 participants; high-quality evidence). Anti-PD1 monoclonal antibodies may result in better toxicity outcomes than anti-CTLA4 monoclonal antibodies (RR 0.70, 95% CI 0.54 to 0.91, 2 studies, 1465 participants; low-quality evidence).Compared to anti-CTLA4 monoclonal antibodies alone, the combination of anti-CTLA4 plus anti-PD1 monoclonal antibodies was associated with better progression-free survival (HR 0.40, 95% CI 0.35 to 0.46, 2 studies, 738 participants; high-quality evidence). There may be no significant difference in toxicity outcomes (RR 1.57, 95% CI 0.85 to 2.92, 2 studies, 764 participants; low-quality evidence) (no data for overall survival were available).The class of small-molecule targeted drugs, BRAF inhibitors (which are active exclusively against BRAF-mutated melanoma), performed better than chemotherapy in terms of overall survival (HR 0.40, 95% CI 0.28 to 0.57, 2 studies, 925 participants; high-quality evidence) and progression-free survival (HR 0.27, 95% CI 0.21 to 0.34, 2 studies, 925 participants; high-quality evidence), and there may be no significant difference in toxicity (RR 1.27, 95% CI 0.48 to 3.33, 2 studies, 408 participants; low-quality evidence).Compared to chemotherapy, MEK inhibitors (which are active exclusively against BRAF-mutated melanoma) may not significantly improve overall survival (HR 0.85, 95% CI 0.58 to 1.25, 3 studies, 496 participants; low-quality evidence), but they probably lead to better progression-free survival (HR 0.58, 95% CI 0.42 to 0.80, 3 studies, 496 participants; moderate-quality evidence). However, MEK inhibitors probably have higher toxicity rates (RR 1.61, 95% CI 1.08 to 2.41, 1 study, 91 participants; moderate-quality evidence).Compared to BRAF inhibitors, the combination of BRAF plus MEK inhibitors was associated with better overall survival (HR 0.70, 95% CI 0.59 to 0.82, 4 studies, 1784 participants; high-quality evidence). BRAF plus MEK inhibitors was also probably better in terms of progression-free survival (HR 0.56, 95% CI 0.44 to 0.71, 4 studies, 1784 participants; moderate-quality evidence), and there appears likely to be no significant difference in toxicity (RR 1.01, 95% CI 0.85 to 1.20, 4 studies, 1774 participants; moderate-quality evidence).Compared to chemotherapy, the combination of chemotherapy plus anti-angiogenic drugs was probably associated with better overall survival (HR 0.60, 95% CI 0.45 to 0.81; moderate-quality evidence) and progression-free survival (HR 0.69, 95% CI 0.52 to 0.92; moderate-quality evidence). There may be no difference in terms of toxicity (RR 0.68, 95% CI 0.09 to 5.32; low-quality evidence). All results for this comparison were based on 324 participants from 2 studies.Network meta-analysis focused on chemotherapy as the common comparator and currently approved treatments for which high- to moderate-quality evidence of efficacy (as represented by treatment effect on progression-free survival) was available (based on the above results) for: biochemotherapy (with both interferon-alpha and interleukin-2); anti-CTLA4 monoclonal antibodies; anti-PD1 monoclonal antibodies; anti-CTLA4 plus anti-PD1 monoclonal antibodies; BRAF inhibitors; MEK inhibitors, and BRAF plus MEK inhibitors. Analysis (which included 19 RCTs and 7632 participants) generated 21 indirect comparisons.The best evidence (moderate-quality evidence) for progression-free survival was found for the following indirect comparisons:• both combinations of immune checkpoint inhibitors (HR 0.30, 95% CI 0.17 to 0.51) and small-molecule targeted drugs (HR 0.17, 95% CI 0.11 to 0.26) probably improved progression-free survival compared to chemotherapy;• both BRAF inhibitors (HR 0.40, 95% CI 0.23 to 0.68) and combinations of small-molecule targeted drugs (HR 0.22, 95% CI 0.12 to 0.39) were probably associated with better progression-free survival compared to anti-CTLA4 monoclonal antibodies;• biochemotherapy (HR 2.81, 95% CI 1.76 to 4.51) probably lead to worse progression-free survival compared to BRAF inhibitors;• the combination of small-molecule targeted drugs probably improved progression-free survival (HR 0.38, 95% CI 0.21 to 0.68) compared to anti-PD1 monoclonal antibodies;• both biochemotherapy (HR 5.05, 95% CI 3.01 to 8.45) and MEK inhibitors (HR 3.16, 95% CI 1.77 to 5.65) were probably associated with worse progression-free survival compared to the combination of small-molecule targeted drugs; and• biochemotherapy was probably associated with worse progression-free survival (HR 2.81, 95% CI 1.54 to 5.11) compared to the combination of immune checkpoint inhibitors.The best evidence (moderate-quality evidence) for toxicity was found for the following indirect comparisons:• combination of immune checkpoint inhibitors (RR 3.49, 95% CI 2.12 to 5.77) probably increased toxicity compared to chemotherapy;• combination of immune checkpoint inhibitors probably increased toxicity (RR 2.50, 95% CI 1.20 to 5.20) compared to BRAF inhibitors;• the combination of immune checkpoint inhibitors probably increased toxicity (RR 3.83, 95% CI 2.59 to 5.68) compared to anti-PD1 monoclonal antibodies; and• biochemotherapy was probably associated with lower toxicity (RR 0.41, 95% CI 0.24 to 0.71) compared to the combination of immune checkpoint inhibitors.Network meta-analysis-based ranking suggested that the combination of BRAF plus MEK inhibitors is the most effective strategy in terms of progression-free survival, whereas anti-PD1 monoclonal antibodies are associated with the lowest toxicity.Overall, the risk of bias of the included trials can be considered as limited. When considering the 122 trials included in this review and the seven types of bias we assessed, we performed 854 evaluations only seven of which (< 1%) assigned high risk to six trials.
Authors' conclusions: We found high-quality evidence that many treatments offer better efficacy than chemotherapy, especially recently implemented treatments, such as small-molecule targeted drugs, which are used to treat melanoma with specific gene mutations. Compared with chemotherapy, biochemotherapy (in this case, chemotherapy combined with both interferon-alpha and interleukin-2) and BRAF inhibitors improved progression-free survival; BRAF inhibitors (for BRAF-mutated melanoma) and anti-PD1 monoclonal antibodies improved overall survival. However, there was no difference between polychemotherapy and monochemotherapy in terms of achieving progression-free survival and overall survival. Biochemotherapy did not significantly improve overall survival and has higher toxicity rates compared with chemotherapy.There was some evidence that combined treatments worked better than single treatments: anti-PD1 monoclonal antibodies, alone or with anti-CTLA4, improved progression-free survival compared with anti-CTLA4 monoclonal antibodies alone. Anti-PD1 monoclonal antibodies performed better than anti-CTLA4 monoclonal antibodies in terms of overall survival, and a combination of BRAF plus MEK inhibitors was associated with better overall survival for BRAF-mutated melanoma, compared to BRAF inhibitors alone.The combination of BRAF plus MEK inhibitors (which can only be administered to people with BRAF-mutated melanoma) appeared to be the most effective treatment (based on results for progression-free survival), whereas anti-PD1 monoclonal antibodies appeared to be the least toxic, and most acceptable, treatment.Evidence quality was reduced due to imprecision, between-study heterogeneity, and substandard reporting of trials. Future research should ensure that those diminishing influences are addressed. Clinical areas of future investigation should include the longer-term effect of new therapeutic agents (i.e. immune checkpoint inhibitors and targeted therapies) on overall survival, as well as the combination of drugs used in melanoma treatment; research should also investigate the potential influence of biomarkers.
Conflict of interest statement
Sandro Pasquali: nothing to declare. Andreas V Hadjinicolaou: nothing to declare. Vanna Chiarion Sileni: nothing to declare. Carlo Riccardo Rossi: nothing to declare. Simone Mocellin: nothing to declare.
Figures

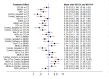
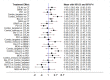
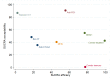
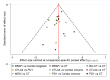











































































Update of
References
References to studies included in this review
Agarwala 1999 {published data only}
-
- Agarwala SS, Ferri W, Gooding W, Kirkwood JM. A phase III randomized trial of dacarbazine and carboplatin with and without tamoxifen in the treatment of patients with metastatic melanoma. Cancer 1999;85(9):1979-84. [PMID: ] - PubMed
Agarwala 2002 {published data only}
-
- Agarwala SS, Glaspy J, O'Day SJ, Mitchell M, Gutheil J, Whitman E, et al. Results from a randomized phase III study comparing combined treatment with histamine dihydrochloride plus interleukin-2 versus interleukin-2 alone in patients with metastatic melanoma. Journal of Clinical Oncology 2002;20(1):125-33. [PMID: ] - PubMed
Atkins 2008 {published data only}
-
- Atkins MB, Hsu J, Lee S, Cohen GI, Flaherty LE, Sosman JA, et al. Phase III trial comparing concurrent biochemotherapy with cisplatin, vinblastine, dacarbazine, interleukin-2, and interferon alfa-2b with cisplatin, vinblastine, and dacarbazine alone in patients with metastatic malignant melanoma (E3695): a trial coordinated by the Eastern Cooperative Oncology Group. Journal of Clinical Oncology 2008;26(35):5748-54. [PMID: ] - PMC - PubMed
Atzpodien 2002 {published data only}
-
- Atzpodien J, Neuber K, Kamanabrou D, Fluck M, Brocker EB, Neumann C, et al. Combination chemotherapy with or without s.c. IL-2 and IFN-alpha: results of a prospectively randomized trial of the Cooperative Advanced Malignant Melanoma Chemoimmunotherapy Group (ACIMM). British Journal of Cancer 2002;86(2):179-84. [PMID: ] - PMC - PubMed
Avril 2004 {published data only}
-
- Avril MF, Aamdal S, Grob JJ, Hauschild A, Mohr P, Bonerandi JJ, et al. Fotemustine compared with dacarbazine in patients with disseminated malignant melanoma: a phase III study. Journal of Clinical Oncology 2004;22(6):1118-25. [PMID: ] - PubMed
Bafaloukos 2005 {published data only}
-
- Bafaloukos D, Tsoutsos D, Kalofonos H, Chalkidou S, Panagiotou P, Linardou E, et al. Temozolomide and cisplatin versus temozolomide in patients with advanced melanoma: a randomized phase II study of the Hellenic Cooperative Oncology Group. Annals of Oncology 2005;16(6):950-7. [PMID: ] - PubMed
Bajetta 1985 {published data only}
-
- Bajetta E, Buzzoni R, Viviani S, Vaglini M, Nava M, Bonadonna G. Prospective randomized trial in advanced malignant melanoma with cis-platinum, vindesine, and etoposide vs. cis-platinum, vindesine, and lomustine. American Journal of Clinical Oncology 1985;8(5):401-5. [PMID: ] - PubMed
Bajetta 1994 {published data only}
-
- Bajetta E, Di Leo A, Zampino MG, Sertoli MR, Comella G, Barduagni M, et al. Multicenter randomized trial of dacarbazine alone or in combination with two different doses and schedules of interferon alfa-2a in the treatment of advanced melanoma. Journal of Clinical Oncology 1994;12(4):806-11. [PMID: ] - PubMed
Bajetta 2006a {published data only}
-
- Bajetta E, Del Vecchio M, Nova P, Fusi A, Daponte A, Sertoli MR, et al. Multicenter phase III randomized trial of polychemotherapy (CVD regimen) versus the same chemotherapy (CT) plus subcutaneous interleukin-2 and interferon-alpha2b in metastatic melanoma. Annals of Oncology 2006;17(4):571-7. [PMID: ] - PubMed
Balch 1984 {published data only}
-
- Balch CM, Murray D, Presant C, Bartolucci AA. Ineffectiveness of adjuvant chemotherapy using DTIC and cyclophosphamide in patients with resectable metastatic melanoma. Surgery 1984;95(4):454-9. [PMID: ] - PubMed
Bedikian 2006 {published data only}
-
- Bedikian AY, Millward M, Pehamberger H, Conry R, Gore M, Trefzer U, et al. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: the Oblimersen Melanoma Study Group. Journal of Clinical Oncology 2006;24(29):4738-45. [PMID: ] - PubMed
Bedikian 2011 {published data only}
Bellett 1976 {published data only}
-
- Bellett RE, Mastrangelo MJ, Laucius JF, Bodurtha AJ. Randomized prospective trial of DTIC (NSC-45388) alone versus BCNU (NSC-409962) plus vincristine (NSC-67574) in the treatment of metastatic malignant melanoma. Cancer Treatment Reports 1976;60(5):595-600. [PMID: ] - PubMed
Beretta 1976 {published data only}
-
- Beretta G, Bonadonna G, Cascinelli N, Morabito A, Veronesi U. Comparative evaluation of three combination regimens for advanced malignant melanoma: results of an international cooperative study. Cancer Treatment Reports 1976;60(1):33-40. [PMID: ] - PubMed
Carter 1975 {published data only}
-
- Carter RD, Krementz ET, Hill GJ. DTIC and combination therapy for metastatic melanoma: a COG cooperative study. Proceedings of the American Association for Cancer Research 1975;16(66):NO.
Carvajal 2014 {published data only}
Chapman 1999 {published data only}
-
- Chapman PB, Einhorn LH, Meyers ML, Saxman S, Destro AN, Panageas KS, et al. Phase III multicenter randomized trial of the Dartmouth regimen versus dacarbazine in patients with metastatic melanoma. Journal of Clinical Oncology 1999;17(9):2745-51. [PMID: ] - PubMed
Chauvergne 1982 {published data only}
-
- Chauvergne J, Bui NB, Cappelaere P, Gary-Bobo J, Guerrin J, Armand JP, et al. [Chemotherapy in advanced malignant melanoma. Results of a controlled trial comparing a combination of dacarbazine (DTIC) and detorubicin with dacarbazine alone] [Chimiotherapie des melanomes malins evolues. Resultats d'un essai controle comparant l'association de detorubicine et de dacarbazine (DTIC) a la dacarbazine seule.]. La Semaine des Hopitaux 1982;58(46):2697-701. [PMID: ] - PubMed
Chiarion Sileni 2001 {published data only}
-
- Chiarion Sileni V, Nortilli R, Aversa SM, Paccagnella A, Medici M, Corti L, et al. Phase II randomized study of dacarbazine, carmustine, cisplatin and tamoxifen versus dacarbazine alone in advanced melanoma patients. Melanoma Research 2001;11(2):189-96. [PMID: ] - PubMed
Chiarion‐Sileni 2011 {published data only}
-
- Chiarion-Sileni V, Guida M, Ridolfi L, Romanini A, Del Bianco P, Pigozzo J, et al. Central nervous system failure in melanoma patients: results of a randomised, multicentre phase 3 study of temozolomide- and dacarbazine- based regimens. British Journal of Cancer 2011;104(12):1816-21. [PMID: ] - PMC - PubMed
Clunie 1980 {published data only}
-
- Clunie GJ, Gough IR, Dury M, Furnival CM, Bolton PM. A trial of imidazole carboxamide and corynebacterium parvum in disseminated melanoma: clinical and immunologic results. Cancer 1980;46(3):475-9. [PMID: ] - PubMed
Cocconi 1992 {published data only}
-
- Cocconi G, Bella M, Calabresi F, Tonato M, Canaletti R, Boni C, et al. Treatment of metastatic malignant melanoma with dacarbazine plus tamoxifen. New England Journal of Medicine 1992;327(8):516-23. [PMID: ] - PubMed
Cocconi 2003 {published data only}
-
- Cocconi G, Passalacqua R, Foladore S, Carlini P, Acito L, Maiello E, et al. Treatment of metastatic malignant melanoma with dacarbazine plus tamoxifen, or vindesine plus tamoxifen: a prospective randomized study. Melanoma Research 2003;13(1):73-9. [PMID: ] - PubMed
Costanza 1972 {published data only}
-
- Costanza ME, Nathanson L, Lenhard R, Wolter J, Colsky J, Oberfield RA, et al. Therapy of malignant melanoma with an imidazole carboxamide and bis-chloroethyl nitrosourea. Cancer 1972;30(6):1457-61. [PMID: ] - PubMed
Costanza 1977 {published data only}
-
- Costanza ME, Nathanson L, Schoenfeld D, Wolter J, Colsky J, Regelson W, et al. Results with methyl-CCNU and DTIC in metastatic melanoma. Cancer 1977;40(3):1010-5. [PMID: ] - PubMed
Costanzi 1982 {published data only}
-
- Costanzi JJ, Al-Sarraf M, Groppe C, Bottomley R, Fabian C, Neidhart J, et al. Combination chemotherapy plus BCG in the treatment of disseminated malignant melanoma: a Southwest Oncology Group Study. Medical and Pediatric Oncology 1982;10(3):251-8. [PMID: ] - PubMed
Cui 2013 {published data only}
Danson 2003 {published data only}
-
- Danson S, Lorigan P, Arance A, Clamp A, Ranson M, Hodgetts J, et al. Randomized phase II study of temozolomide given every 8 hours or daily with either interferon alfa-2b or thalidomide in metastatic malignant melanoma. Journal of Clinical Oncology 2003;21(13):2551-7. [PMID: ] - PubMed
Daponte 2013 {published data only}
Dorval 1999 {published data only}
-
- Dorval T, Negrier S, Chevreau C, Avril MF, Baume D, Cupissol D, et al. Randomized trial of treatment with cisplatin and interleukin-2 either alone or in combination with interferon-alpha-2a in patients with metastatic melanoma: a Federation Nationale des Centres de Lutte Contre le Cancer Multicenter, parallel study. Cancer 1999;85(5):1060-6. [PMID: ] - PubMed
Dummer 2006 {published data only}
-
- Dummer R, Garbe C, Thompson JA, Eggermont AM, Yoo K, Maier T, et al. Randomized dose-escalation study evaluating peginterferon alfa-2a in patients with metastatic malignant melanoma. Journal of Clinical Oncology 2006;24(7):1188-94. [PMID: ] - PubMed
Eigentler 2008 {published data only}
-
- Eigentler TK, Radny P, Hauschild A, Gutzmer R, Linse R, Pfohler C, et al. Adjuvant treatment with vindesine in comparison to observation alone in patients with metastasized melanoma after complete metastasectomy: a randomized multicenter trial of the German Dermatologic Cooperative Oncology Group. Melanoma Research 2008;18(5):353-8. [PMID: ] - PubMed
Eisen 2010 {published data only}
-
- Eisen T, Trefzer U, Hamilton A, Hersey P, Millward M, Knight RD, et al. Results of a multicenter, randomized, double-blind phase 2/3 study of lenalidomide in the treatment of pretreated relapsed or refractory metastatic malignant melanoma. Cancer 2010;116(1):146-54. [PMID: ] - PubMed
Eton 2002 {published data only}
-
- Eton O, Legha SS, Bedikian AY, Lee JJ, Buzaid AC, Hodges C, et al. Sequential biochemotherapy versus chemotherapy for metastatic melanoma: results from a phase III randomized trial. Journal of Clinical Oncology 2002;20(8):2045-52. [PMID: ] - PubMed
Falkson 1991 {published data only}
-
- Falkson CI, Falkson G, Falkson HC. Improved results with the addition of interferon alfa-2b to dacarbazine in the treatment of patients with metastatic malignant melanoma. Journal of Clinical Oncology 1991;9(8):1403-8. [PMID: ] - PubMed
Falkson 1995 {published data only}
-
- Falkson CI. Experience with interferon alpha 2b combined with dacarbazine in the treatment of metastatic malignant melanoma. Medical Oncology 1995;12(1):35-40. [PMID: ] - PubMed
Falkson 1998 {published data only}
-
- Falkson CI, Ibrahim J, Kirkwood JM, Coates AS, Atkins MB, Blum RH. Phase III trial of dacarbazine versus dacarbazine with interferon alpha-2b versus dacarbazine with tamoxifen versus dacarbazine with interferon alpha-2b and tamoxifen in patients with metastatic malignant melanoma: an Eastern Cooperative Oncology Group study. Journal of Clinical Oncology 1998;16(5):1743-51. [PMID: ] - PubMed
Flaherty 2001 {published data only}
-
- Flaherty LE, Atkins M, Sosman J, Weiss G, Clark JI, Margolin K, et al. Outpatient biochemotherapy with interleukin-2 and interferon alfa-2b in patients with metastatic malignant melanoma: results of two phase II cytokine working group trials. Journal of Clinical Oncology 2001;19(13):3194-202. [PMID: ] - PubMed
Flaherty 2012a {published data only}
Flaherty 2012b {published data only}
-
- Flaherty KT, Robert C, Hersey P, Nathan P, Garbe C, Milhem M, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. New England Journal of Medicine 2012;367(2):107-14. [PMID: ] - PubMed
Flaherty 2013a {published data only}
Glaspy 2009 {published data only}
-
- Glaspy J, Atkins MB, Richards JM, Agarwala SS, O'Day S, Knight RD, et al. Results of a multicenter, randomized, double-blind, dose-evaluating phase 2/3 study of lenalidomide in the treatment of metastatic malignant melanoma. Cancer 2009;115(22):5228-36. [PMID: ] - PubMed
Glover 2003 {published data only}
-
- Glover D, Ibrahim J, Kirkwood J, Glick J, Karp D, Stewart J, et al. Phase II randomized trial of cisplatin and WR-2721 versus cisplatin alone for metastatic melanoma: an Eastern Cooperative Oncology Group Study (E1686). Melanoma Research 2003;13(6):619-26. [PMID: ] - PubMed
Gorbonova 2000 {published data only}
-
- Gorbonova VA, Egorov GN, Perevodchikova NI, Orel NF. Combined chemotherapy with or without interferon alpha N1 (IFN) for advanced malignant melanoma--a randomized pilot phase III study. Gan to Kagaku Ryoho. Cancer & Chemotherapy 2000;27(Suppl 2):310-4. [PMID: ] - PubMed
Gough 1978 {published data only}
-
- Gough IR, Bolton PM, Clunie GJ, Burnett W. Chemoimmunotherapy in disseminated melanoma and colorectal carcinoma. Australian and New Zealand Journal of Surgery 1978;48(3):296-300. [PMID: ] - PubMed
Gupta 2014 {published data only}
-
- Gupta A, Love S, Schuh A, Shanyinde M, Larkin JM, Plummer R, et al. DOC-MEK: a double-blind randomized phase II trial of docetaxel with or without selumetinib in wild-type BRAF advanced melanoma. Annals of Oncology 2014;25(5):968-74. [PMID: ] - PubMed
Hamid 2014 {published data only}
-
- Hamid O, Ilaria R, Garbe C, Wolter P, Maio M, Hutson TE, et al. A randomized, open-label clinical trial of tasisulam sodium versus paclitaxel as second-line treatment in patients with metastatic melanoma. Cancer 2014;120(13):2016-24. [PMID: ] - PubMed
Hauschild 2001 {published data only}
-
- Hauschild A, Garbe C, Stolz W, Ellwanger U, Seiter S, Dummer R, et al. Dacarbazine and interferon alpha with or without interleukin 2 in metastatic melanoma: a randomized phase III multicentre trial of the Dermatologic Cooperative Oncology Group (DeCOG). British Journal of Cancer 2001;84(8):1036-42. [PMID: ] - PMC - PubMed
Hauschild 2009a {published data only}
-
- Hauschild A, Agarwala SS, Trefzer U, Hogg D, Robert C, Hersey P, et al. Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. Journal of Clinical Oncology 2009;27(17):2823-30. [PMID: ] - PubMed
Hauschild 2012 {published data only}
-
- Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012;380(9839):358-65. [PMID: ] - PubMed
Hersh 2015 {published data only}
Hodi 2010a {published data only}
Hodi 2014 {published data only}
Hofmann 2011 {published data only}
-
- Hofmann MA, Hauschild A, Mohr P, Garbe C, Weichenthal M, Trefzer U, et al. Prospective evaluation of supportive care with or without CVD chemotherapy as a second-line treatment in advanced melanoma by patient's choice: a multicentre Dermatologic Cooperative Oncology Group trial. Melanoma Research 2011;21(6):516-23. [PMID: ] - PubMed
Jelic 2002 {published data only}
-
- Jelic S, Babovic N, Kovcin V, Milicevic N, Milanovic N, Popov I, et al. Comparison of the efficacy of two different dosage dacarbazine-based regimens and two regimens without dacarbazine in metastatic melanoma: a single-centre randomized four-arm study. Melanoma Research 2002;12(1):91-8. [PMID: ] - PubMed
Johnston 1998 {published data only}
-
- Johnston SR, Constenla DO, Moore J, Atkinson H, A'Hern RP, Dadian G, et al. Randomized phase II trial of BCDT [carmustine (BCNU), cisplatin, dacarbazine (DTIC) and tamoxifen] with or without interferon alpha (IFN-alpha) and interleukin (IL-2) in patients with metastatic melanoma. British Journal of Cancer 1998;77(8):1280-6. [PMID: ] - PMC - PubMed
Kaufmann 2005 {published data only}
-
- Kaufmann R, Spieth K, Leiter U, Mauch C, den Driesch P, Vogt T, et al. Temozolomide in combination with interferon-alfa versus temozolomide alone in patients with advanced metastatic melanoma: a randomized, phase III, multicenter study from the Dermatologic Cooperative Oncology Group. Journal of Clinical Oncology 2005;23(35):9001-7. [PMID: ] - PubMed
Kefford 2010 {published data only}
Keilholz 1997 {published data only}
-
- Keilholz U, Goey SH, Punt CJ, Proebstle TM, Salzmann R, Scheibenbogen C, et al. Interferon alfa-2a and interleukin-2 with or without cisplatin in metastatic melanoma: a randomized trial of the European Organization for Research and Treatment of Cancer Melanoma Cooperative Group. Journal of Clinical Oncology 1997;15(7):2579-88. [PMID: ] - PubMed
Keilholz 2005 {published data only}
-
- Keilholz U, Punt CJ, Gore M, Kruit W, Patel P, Lienard D, et al. Dacarbazine, cisplatin, and interferon-alfa-2b with or without interleukin-2 in metastatic melanoma: a randomized phase III trial (18951) of the European Organisation for Research and Treatment of Cancer Melanoma Group. Journal of Clinical Oncology 2005;23(27):6747-55. [PMID: ] - PubMed
Kim 2012 {published data only}
-
- Kim KB, Sosman JA, Fruehauf JP, Linette GP, Markovic SN, McDermott DF, et al. BEAM: a randomized phase II study evaluating the activity of bevacizumab in combination with carboplatin plus paclitaxel in patients with previously untreated advanced melanoma. Journal of Clinical Oncology 2012;30(1):34-41. [PMID: ] - PMC - PubMed
Kirkwood 1990 {published data only}
-
- Kirkwood JM, Ernstoff MS, Giuliano A, Gams R, Robinson WA, Costanzi J, et al. Interferon alpha-2a and dacarbazine in melanoma. Journal of the National Cancer Institute 1990;82(12):1062-3. [PMID: ] - PubMed
Kogoniia 1981 {published data only}
-
- Kogoniia LM, Moroz LV, Perevodchikova NI, Platinskii LV, Borisov AI. Comparison of the efficacy of imidazole-carboxamide and of a combination of nitrosomethylurea, vincristine and dactinomycin in disseminated melanoma [Sravnenie effiktivnosti imidazol-karboksamida i kombinatsii nitrozometilmocheviny, vinkristina, daktinomitsina pri disseminirovannoi melanome.]. Voprosy Onkologii 1981;27(4):16-21. [PMID: ] - PubMed
Kokoschka 1978 {published data only}
-
- Kokoschka EM, Luger T, Micksche M. Immuno-chemotherapy in patients with disseminated metastasizing stage III melanoma. Randomized study with methyl-CCNU versus C. parvum plus methyl-CCNU [Immuno-Chemotherapie bei Patienten mit disseminiert metastasierendem Melanom Stadium III Randomisierte Studie mit Methyl-CCNU versus C. parvum plus Methyl-CCNU]. Onkologie 1978;1(3):98-103. [PMID: ] - PubMed
Larkin 2014 {published data only}
-
- Larkin J, Ascierto PA, Dréno B, Atkinson V, Liszkay G, Maio M, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. New England Journal of Medicine 2014;371(20):1867-76. [PMID: ] - PubMed
Larkin 2015 {published data only}
Lawson 2015 {published data only}
-
- Lawson DH, Lee S, Zhao F, Tarhini AA, Margolin KA, Ernstoff MS, et al. Randomized, placebo-controlled, phase III trial of yeast-derived granulocyte-macrophage colony-stimulating factor (GM-CSF) versus peptide vaccination versus GM-CSF plus peptide vaccination versus placebo in patients with no evidence of disease after complete surgical resection of locally advanced and/or stage IV melanoma: A trial of the Eastern Cooperative Oncology Group-American College of Radiology Imaging Network Cancer Research Group (E4697). Journal of Clinical Oncology 2015;33(34):4066-76. [PMID: ] - PMC - PubMed
Legha 1996 {published data only}
-
- Legha SS, Ring S, Bedikian A, Plager C, Eton O, Buzaid AC, et al. Treatment of metastatic melanoma with combined chemotherapy containing cisplatin, vinblastine and dacarbazine (CVD) and biotherapy using interleukin-2 and interferon-alpha. Annals of Oncology 1996;7(8):827-35. [PMID: ] - PubMed
Long 2015 {published data only}
-
- Long GV, Stroyakovskiy D, Gogas H, Levchenko E, Braud F, Larkin J, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet 2015;386(9992):444-51. [PMID: ] - PubMed
Lopez 1984 {published data only}
-
- Lopez M, Perno CF, Di Lauro L, Papaldo P, Ganzina F, Barduagni A. Controlled study of DTIC versus DTIC plus epirubicin in metastatic malignant melanoma. Investigational New Drugs 1984;2(3):319-22. [PMID: ] - PubMed
Luikart 1984 {published data only}
-
- Luikart SD, Kennealey GT, Kirkwood JM. Randomized phase III trial of vinblastine, bleomycin, and cis-dichlorodiammine-platinum versus dacarbazine in malignant melanoma. Journal of Clinical Oncology 1984;2(3):164-8. [PMID: ] - PubMed
Maio 2010 {published data only}
-
- Maio M, Mackiewicz A, Testori A, Trefzer U, Ferraresi V, Jassem J, et al. Large randomized study of thymosin alpha 1, interferon alfa, or both in combination with dacarbazine in patients with metastatic melanoma. Journal of Clinical Oncology 2010;28(10):1780-7. [PMID: ] - PubMed
Mastrangelo 1979 {published data only}
-
- Mastrangelo MJ, Bellet RE, Berd D. A phase III comparison of methyl-CCNU + vincristine with or without BCG + allogeneic tumor cells in metastatic melanoma. Cancer Immunology, Immunotherapy 1979;6(4):231-6. [EMBASE: 1980000708] [Mastrangelo 1979]
McArthur 2014 {published data only}
McDermott 2008 {published data only}
-
- McDermott DF, Sosman JA, Gonzalez R, Hodi FS, Linette GP, Richards J, et al. Double-blind randomized phase II study of the combination of sorafenib and dacarbazine in patients with advanced melanoma: a report from the 11715 Study Group. Journal of Clinical Oncology 2008;26(13):2178-85. [PMID: ] - PubMed
Middleton 2000 {published data only}
-
- Middleton MR, Grob JJ, Aaronson N, Fierlbeck G, Tilgen W, Seiter S, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. Journal of Clinical Oncology 2000;18(1):158-66. [PMID: ] - PubMed
Middleton 2007 {published data only}
-
- Middleton M, Hauschild A, Thomson D, Anderson R, Burdette-Radoux S, Gehlsen K, et al. Results of a multicenter randomized study to evaluate the safety and efficacy of combined immunotherapy with interleukin-2, interferon-{alpha}2b and histamine dihydrochloride versus dacarbazine in patients with stage IV melanoma. Annals of Oncology 2007;18(10):1691-7. [PMID: ] - PubMed
Middleton 2015 {published data only}
-
- Middleton MR, Friedlander P, Hamid O, Daud A, Plummer R, Falotico N, et al. Randomized phase II study evaluating veliparib (ABT-888) with temozolomide in patients with metastatic melanoma. Annals of Oncology 2015;26(10):2173-9. [PMID: ] - PubMed
Miller 1989 {published data only}
-
- Miller RL, Steis RG, Clark JW, Smith JW 2nd, Crum E, McKnight JE, et al. Randomized trial of recombinant alpha 2b-interferon with or without indomethacin in patients with metastatic malignant melanoma. Cancer Research 1989;49(7):1871-6. [PMID: ] - PubMed
Moon 1975 {published data only}
-
- Moon JH, Gailani S, Cooper MR, Hayes DM, Rege VB, Blom J, et al. Comparison of the combination of 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) and vincristine with two dose schedules of 5-(3,3-dimethyl-1-triazino) imidazole 4-carboxamide (DTIC) in the treatment of disseminated malignant melanoma. Cancer 1975;35(2):368-71. [PMID: ] - PubMed
Newlands 1976 {published data only}
O'Day 2009 {published data only}
-
- O'Day S, Gonzalez R, Lawson D, Weber R, Hutchins L, Anderson C, et al. Phase II, randomized, controlled, double-blinded trial of weekly elesclomol plus paclitaxel versus paclitaxel alone for stage IV metastatic melanoma. Journal of Clinical Oncology 2009;27(32):5452-8. [PMID: ] - PubMed
O'Day 2011 {published data only}
O'Day 2013 {published data only}
-
- O'Day SJ, Eggermont AM, Chiarion-Sileni V, Kefford R, Grob JJ, Mortier L, et al. Final results of phase III SYMMETRY study: randomized, double-blind trial of elesclomol plus paclitaxel versus paclitaxel alone as treatment for chemotherapy-naive patients with advanced melanoma. Journal of Clinical Oncology 2013;31(9):1211-8. [PMID: ] - PubMed
Patel 2011 {published data only}
-
- Patel PM, Suciu S, Mortier L, Kruit WH, Robert C, Schadendorf D, et al. Extended schedule, escalated dose temozolomide versus dacarbazine in stage IV melanoma: final results of a randomised phase III study (EORTC 18032). European Journal of Cancer 2011;47(10):1476-83. [PMID: ] - PubMed
Postow 2015 {published data only}
Presant 1979 {published data only}
-
- Presant CA, Bartolucci AA, Smalley RV, Vogler WR. Cyclophosphamide plus 5-(3,3-dimethyl-1-triazeno)-imidazole-4-carboxamide (DTIC) with or without Corynebacterium parvum in metastatic malignant melanoma. Cancer 1979;44(3):899-905. [PMID: ] - PubMed
Presant 1982 {published data only}
-
- Presant CA, Bartolucci AA, Balch C, Troner M. A randomized comparison of cyclophosphamide, DTIC with or without piperazinedione in metastatic malignant melanoma. Cancer 1982;49(7):1355-7. [PMID: ] - PubMed
Punt 2006 {published data only}
-
- Punt CJ, Suciu S, Gore MA, Koller J, Kruit WH, Thomas J, et al. Chemoimmunotherapy with dacarbazine, cisplatin, interferon-alpha2b and interleukin-2 versus two cycles of dacarbazine followed by chemoimmunotherapy in patients with metastatic melanoma: a randomised phase II study of the European Organization for Research and Treatment of Cancer Melanoma Group. European Journal of Cancer 2006;42(17):2991-5. [PMID: ] - PubMed
Ramseur 1978 {published data only}
-
- Ramseur WL, Richards F 2nd, Muss HB, Rhyne L, Cooper MR, White DR, et al. Chemoimmunotherapy for disseminated malignant melanoma: a prospective randomized study. Cancer Treatment Reports 1978;62(7):1085-7. [PMID: ] - PubMed
Ranson 2007 {published data only}
-
- Ranson M, Hersey P, Thompson D, Beith J, McArthur GA, Haydon A, et al. Randomized trial of the combination of lomeguatrib and temozolomide compared with temozolomide alone in chemotherapy naive patients with metastatic cutaneous melanoma. Journal of Clinical Oncology 2007;25(18):2540-5. [PMID: ] - PubMed
Reichle 2007 {published data only}
-
- Reichle A, Vogt T, Coras B, Terheyden P, Neuber K, Trefzer U, et al. Targeted combined anti-inflammatory and angiostatic therapy in advanced melanoma: a randomized phase II trial. Melanoma Research 2007;17(6):360-4. [PMID: ] - PubMed
Ribas 2013 {published data only}
Ribas 2015 {published data only}
Richtig 2004 {published data only}
-
- Richtig E, Hofmann-Wellenhof R, Pehamberger H, Forstinger Ch, Wolff K, Mischer P, et al. Temozolomide and interferon alpha 2b in metastatic melanoma stage IV. British Journal of Dermatology 2004;151(1):91-8. [PMID: ] - PubMed
Ridolfi 2002a {published data only}
-
- Ridolfi R, Chiarion-Sileni V, Guida M, Romanini A, Labianca R, Freschi A, et al. Cisplatin, dacarbazine with or without subcutaneous interleukin-2, and interferon alpha-2b in advanced melanoma outpatients: results from an Italian multicenter phase III randomized clinical trial. Journal of Clinical Oncology 2002;20(6):1600-7. [PMID: ] - PubMed
Ringborg 1989 {published data only}
-
- Ringborg U, Rudenstam CM, Hansson J, Hafstrom L, Stenstam B, Strander H. Dacarbazine versus dacarbazine-vindesine in disseminated malignant melanoma: a randomized phase II study. Medical Oncology and Tumor Pharmacotherapy 1989;6(4):285-9. [PMID: ] - PubMed
Robert 2011 {published data only}
-
- Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. New England Journal of Medicine 2011;364(26):2517-26. [PMID: ] - PubMed
Robert 2013 {published data only}
-
- Robert C, Dummer R, Gutzmer R, Lorigan P, Kim KB, Nyakas M, et al. Selumetinib plus dacarbazine versus placebo plus dacarbazine as first-line treatment for BRAF-mutant metastatic melanoma: a phase 2 double-blind randomised study. Lancet Oncology 2013;14(8):733-40. [PMID: ] - PubMed
Robert 2015 {published data only}
-
- Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. New England Journal of Medicine 2015;372(1):30-9. [PMID: ] - PubMed
Robert 2015a {published data only}
-
- Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. New England Journal of Medicine 2015;372(4):320-30. [PMID: ] - PubMed
Robert 2015b {published data only}
-
- Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. New England Journal of Medicine 2015;372(26):2521-32. [PMID: 25891173] - PubMed
Robidoux 1982 {published data only}
-
- Robidoux A, Gutterman JU, Bodey GP Sr, Hersh EM. Actinomycin-D plus 5-(3,3-dimethyl-1-triazeno)-imidazole-4-carboxamine (DTIC) with or without intravenous Corynebacterium parvum in metastatic malignant melanoma. Cancer 1982;49(11):2246-51. [PMID: ] - PubMed
Rosenberg 1999 {published data only}
-
- Rosenberg SA, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, et al. Prospective randomized trial of the treatment of patients with metastatic melanoma using chemotherapy with cisplatin, dacarbazine, and tamoxifen alone or in combination with interleukin-2 and interferon alfa-2b. Journal of Clinical Oncology 1999;17(3):968-75. [PMID: ] - PubMed
Rusthoven 1996 {published data only}
-
- Rusthoven JJ, Quirt IC, Iscoe NA, McCulloch PB, James KW, Lohmann RC, et al. Randomized, double-blind, placebo-controlled trial comparing the response rates of carmustine, dacarbazine, and cisplatin with and without tamoxifen in patients with metastatic melanoma. National Cancer Institute of Canada Clinical Trials Group. Journal of Clinical Oncology 1996;14(7):2083-90. [PMID: ] - PubMed
Schadendorf 2006 {published data only}
-
- Schadendorf D, Ugurel S, Schuler-Thurner B, Nestle FO, Enk A, Brocker EB, et al. Dacarbazine (DTIC) versus vaccination with autologous peptide-pulsed dendritic cells (DC) in first-line treatment of patients with metastatic melanoma: a randomized phase III trial of the DC study group of the DeCOG. Annals of Oncology 2006;17(4):563-70. [PMID: ] - PubMed
Schwartzentruber 2011a {published data only}
Sertoli 1999 {published data only}
-
- Sertoli MR, Queirolo P, Bajetta E, Del Vecchio M, Comella G, Barduagni L, et al. Multi-institutional phase II randomized trial of integrated therapy with cisplatin, dacarbazine, vindesine, subcutaneous interleukin-2, interferon alpha2a and tamoxifen in metastatic melanoma. BREMIM (Biological Response Modifiers in Melanoma). Melanoma Research 1999;9(5):503-9. [PMID: ] - PubMed
Sparano 1993 {published data only}
-
- Sparano JA, Fisher RI, Sunderland M, Margolin K, Ernest ML, Sznol M, et al. Randomized phase III trial of treatment with high-dose interleukin-2 either alone or in combination with interferon alfa-2a in patients with advanced melanoma. Journal of Clinical Oncology 1993;11(10):1969-77. [PMID: ] - PubMed
Testori 2008 {published data only}
-
- Testori A, Richards J, Whitman E, Mann GB, Lutzky J, Camacho L, et al. Phase III comparison of vitespen, an autologous tumor-derived heat shock protein gp96 peptide complex vaccine, with physician's choice of treatment for stage IV melanoma: the C-100-21 Study Group. Journal of Clinical Oncology 2008;26(6):955-62. [PMID: ] - PubMed
Thatcher 1986 {published data only}
-
- Thatcher N, Wagstaff J, Mene A, Smith D, Orton C, Craig P. Corynebacterium parvum followed by chemotherapy (actinomycin D and DTIC) compared with chemotherapy alone for metastatic malignant melanoma. European Journal of Cancer & Clinical Oncology 1986;22(8):1009-14. [PMID: ] - PubMed
Thomson 1993 {published data only}
-
- Thomson DB, Adena M, McLeod GR, Hersey P, Gill PG, Coates AS, et al. Interferon-alpha 2a does not improve response or survival when combined with dacarbazine in metastatic malignant melanoma: results of a multi-institutional Australian randomized trial. Melanoma Research 1993;3(2):133-8. [PMID: ] - PubMed
Veronesi 1984 {published data only}
-
- WHO. Controlled study with imidazole carboxamide (DTIC), DTIC + bacillus Calmette-Guerin (BCG), and DTIC + corynebacterium parvum in advanced malignant melanoma. W.H.O. Collaborating Centres for Evaluation of Methods of Diagnosis and Treatment of Melanoma. Tumori 1984;70(1):41-8. [PMID: ] - PubMed
Verschraegen 1993 {published data only}
-
- Verschraegen CF, Legha SS, Hersh EM, Plager C, Papadopoulos N, Burgess MA. Phase II study of vindesine and dacarbazine with or without non-specific stimulation of the immune system in patients with metastatic melanoma. European Journal of Cancer 1993;29A(5):708-11. [PMID: ] - PubMed
Vorobiof 1994 {published data only}
-
- Vorobiof DA, Bezwoda WR. A randomised trial of vindesine plus interferon-alpha 2b compared with interferon-alpha 2b or vindesine alone in the treatment of advanced malignant melanoma. European Journal of Cancer 1994;30A(6):797-800. [PMID: ] - PubMed
Vuoristo 2005 {published data only}
-
- Vuoristo MS, Hahka-Kemppinen M, Parvinen LM, Pyrhonen S, Seppa H, Korpela M, et al. Randomized trial of dacarbazine versus bleomycin, vincristine, lomustine and dacarbazine (BOLD) chemotherapy combined with natural or recombinant interferon-alpha in patients with advanced melanoma. Melanoma Research 2005;15(4):291-6. [PMID: ] - PubMed
Weber 2009 {published data only}
-
- Weber JS, Zarour H, Redman B, Trefzer U, O'Day S, den Eertwegh AJ, et al. Randomized phase 2/3 trial of CpG oligodeoxynucleotide PF-3512676 alone or with dacarbazine for patients with unresectable stage III and IV melanoma. Cancer 2009;115(17):3944-54. [PMID: ] - PubMed
Weber 2015 {published data only}
-
- Weber JS, D'Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncology 2015;16(4):375-84. [PMID: ] - PubMed
Wittes 1978 {published data only}
-
- Wittes RE, Wittes JT, Golbey RB. Combination chemotherapy in metastatic malignant melanoma: a randomized study of three DTIC-containing combination. Cancer 1978;41(2):415-21. [PMID: ] - PubMed
Wolchok 2010 {published data only}
-
- Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncology 2010;11(2):155-64. [PMID: ] - PubMed
Young 2001 {published data only}
-
- Young AM, Marsden J, Goodman A, Burton A, Dunn JA. Prospective randomized comparison of dacarbazine (DTIC) versus DTIC plus interferon-alpha (IFN-alpha) in metastatic melanoma. Clinical Oncology 2001;13(6):458-65. [PMID: ] - PubMed
Zimpfer‐Rechner 2003 {published data only}
-
- Zimpfer-Rechner C, Hofmann U, Figl R, Becker JC, Trefzer U, Keller I, et al. Randomized phase II study of weekly paclitaxel versus paclitaxel and carboplatin as second-line therapy in disseminated melanoma: a multicentre trial of the Dermatologic Co-operative Oncology Group (DeCOG). Melanoma Research 2003;13(5):531-6. [PMID: ] - PubMed
References to studies excluded from this review
Asemissen 2005 {published data only}
-
- Asemissen AM, Scheibenbogen C, Letsch A, Hellstrand K, Thoren F, Gehlsen K, et al. Addition of histamine to interleukin 2 treatment augments type 1 T-cell responses in patients with melanoma in vivo: immunologic results from a randomized clinical trial of interleukin 2 with or without histamine (MP 104). Clinical Cancer Research 2005;11(1):290-7. [PMID: ] - PubMed
Atzpodien 1995 {published data only}
-
- Atzpodien J, Lopez Hanninen E, Kirchner H, Franzke A, Korfer A, Volkenandt M, et al. Chemoimmunotherapy of advanced malignant melanoma: sequential administration of subcutaneous interleukin-2 and interferon-alpha after intravenous dacarbazine and carboplatin or intravenous dacarbazine, cisplatin, carmustine and tamoxifen. European Journal of Cancer 1995;31A(6):876-81. [PMID: ] - PubMed
Bleehen 1995 {published data only}
-
- Bleehen NM, Newlands ES, Lee SM, Thatcher N, Selby P, Calvert AH, et al. Cancer Research Campaign phase II trial of temozolomide in metastatic melanoma. Journal of Clinical Oncology 1995;13(4):910-3. [PMID: ] - PubMed
Buchbinder 2015 {published data only}
-
- Buchbinder EI, Sosman JA, Lawrence DP, McDermott DF, Ramaiya NH, Van den Abbeele AD, et al. Phase 2 study of sunitinib in patients with metastatic mucosal or acral melanoma. Cancer 2015;121(22):4007-15. [PMID: ] - PubMed
Bukowski 1983 {published data only}
-
- Bukowski RM, Deodhar S, Hewlett JS, Greenstreet R. Randomized controlled trial of transfer factor in Stage II malignant melanoma. Cancer 1983;51(2):269-72. [PMID: ] - PubMed
Cashin 2008 {published data only}
-
- Cashin RP, Lui P, Machado M, Hemels ME, Corey-Lisle PK, Einarson TR. Advanced cutaneous malignant melanoma: a systematic review of economic and quality-of-life studies. Value in Health 2008;11(2):259-71. [PMID: ] - PubMed
Cormier 1997 {published data only}
-
- Cormier JN, Hurst R, Vasselli J, Lee D, Kim CJ, McKee M, et al. A prospective randomized evaluation of the prophylactic use of low-dose dopamine in cancer patients receiving interleukin-2. Journal of Immunotherapy 1997;20(4):292-300. [PMID: ] - PubMed
Curl 2014 {published data only}
Downey 2007 {published data only}
Hill 1984 {published data only}
-
- Hill GJ 2nd, Krementz ET, Hill HZ. Dimethyl triazeno imidazole carboxamide and combination therapy for melanoma. IV. Late results after complete response to chemotherapy (Central Oncology Group protocols 7130, 7131, and 7131A). Cancer 1984;53(6):1299-305. [PMID: ] - PubMed
Hughes 2016 {published data only}
-
- Hughes MS, Zager J, Faries M, Alexander HR, Royal RE, Wood B, et al. Results of a randomized controlled multicenter phase III trial of percutaneous hepatic perfusion compared with best available care for patients with melanoma liver metastases. Annals of Surgical Oncology 2016;23(4):1309-19. [PMID: ] - PMC - PubMed
Hwu 2009 {published data only}
-
- Hwu P. Promising results from phase III clinical trial of a peptide vaccine for advanced melanoma. Immunotherapy 2009;1(4):521.
Kaufman 2010 {published data only}
-
- Kaufman HL, Bines SD. OPTIM trial: a Phase III trial of an oncolytic herpes virus encoding GM-CSF for unresectable stage III or IV melanoma. Future Oncology 2010;6(6):941-9. [PMID: ] - PubMed
Kleeberg 1982 {published data only}
-
- Kleeberg UR, Mulder JH, Rumke P, Thomas D, Rozencweig M. N-(phosphonacetyl)-L-aspartate (PALA) in advanced malignant melanoma: a phase II trial of the EORTC Malignant Melanoma Cooperative Group. European Journal of Cancer & Clinical Oncology 1982;18(8):723-6. [PMID: ] - PubMed
Lattanzi 1995 {published data only}
-
- Lattanzi SC, Tosteson T, Chertoff J, Maurer LH, O'Donnell J, LeMarbre PJ, et al. Dacarbazine, cisplatin and carmustine, with or without tamoxifen, for metastatic melanoma: 5-year follow-up. Melanoma Research 1995;5(5):365-9. [PMID: ] - PubMed
McDermott 2013 {published data only}
-
- McDermott D, Haanen J, Chen TT, Lorigan P, O'Day S. Efficacy and safety of ipilimumab in metastatic melanoma patients surviving more than 2 years following treatment in a phase III trial (MDX010-20). Annals of Oncology 2013;24(10):2694-8. [PMID: ] - PubMed
Mornex 2003 {published data only}
-
- Mornex F, Thomas L, Mohr P, Hauschild A, Delaunay MM, Lesimple T, et al. A prospective randomized multicentre phase III trial of fotemustine plus whole brain irradiation versus fotemustine alone in cerebral metastases of malignant melanoma. Melanoma Research 2003;13(1):97-103. [PMID: ] - PubMed
Quirt 1983 {published data only}
-
- Quirt IC, De Boer G, Kersey PA, Baker MA, Bodurtha AJ, Norvell ST, et al. Randomized controlled trial of adjuvant chemoimmunotherapy with DTIC and BCG after complete excision of primary melanoma with a poor prognosis or melanoma metastases. Canadian Medical Association Journal 1983;128(8):929-33. [PMID: 6339024 ] - PMC - PubMed
Richards 1999 {published data only}
-
- Richards JM, Gale D, Mehta N, Lestingi T. Combination of chemotherapy with interleukin-2 and interferon alfa for the treatment of metastatic melanoma. Journal of Clinical Oncology 1999;17(2):651-7. [PMID: ] - PubMed
Spieth 2008 {published data only}
-
- Spieth K, Kaufmann R, Dummer R, Garbe C, Becker JC, Hauschild A, et al. Temozolomide plus pegylated interferon alfa-2b as first-line treatment for stage IV melanoma: a multicenter phase II trial of the Dermatologic Cooperative Oncology Group (DeCOG). Annals of Oncology 2008;19(4):801-6. [PMID: ] - PubMed
Van Dyk 1975 {published data only}
-
- Van Dyk JJ, Falkson G. A clinical trial of procarbazine plus vincristine plus bis-chloroethyl-nitrosourea plus imidazole carboxamide dimethyl triazeno in metastatic malignant melanoma. Medical and Pediatric Oncology 1975;1(2):107-11. [PMID: ] - PubMed
Varker 2007 {published data only}
-
- Varker KA, Biber JE, Kefauver C, Jensen R, Lehman A, Young D, et al. A randomized phase 2 trial of bevacizumab with or without daily low-dose interferon alfa-2b in metastatic malignant melanoma. Annals of Surgical Oncology 2007;14(8):2367-76. [PMID: ] - PubMed
Weber 2013 {published data only}
-
- Weber JS, Dummer R, Pril V, Lebbe C, Hodi FS. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer 2013;119(9):1675-82. [PMID: ] - PubMed
Yang 1995 {published data only}
-
- Yang JC, Topalian SL, Schwartzentruber DJ, Parkinson DR, Marincola FM, Weber JS, et al. The use of polyethylene glycol-modified interleukin-2 (PEG-IL-2) in the treatment of patients with metastatic renal cell carcinoma and melanoma. A phase I study and a randomized prospective study comparing IL-2 alone versus IL-2 combined with PEG-IL-2. Cancer 1995;76(4):687-94. [PMID: ] - PubMed
References to ongoing studies
NCT01280565 {published data only}
-
- NCT01280565. A phase 3 study to compare efficacy and safety of masitinib to dacarbazine in the treatment of patients with non-resectable or metastatic stage 3 or stage 4 melanoma carrying a mutation in the juxta membrane domain of C-Kit. clinicaltrials.gov/ct2/show/NCT01280565 (first received 6 August 2010).
NCT01515189 {published data only}
-
- NCT01515189. Phase 3 trial in subjects with metastatic melanoma comparing 3 mg/kg ipilimumab versus 10 mg/kg ipilimumab. clinicaltrials.gov/ct2/show/NCT01515189 (first received 18 January 2012).
NCT01763164 {published data only}
-
- NCT01763164. Study comparing the efficacy of MEK162 versus dacarbazine in unresectable or metastatic NRAS mutation-positive melanoma. clinicaltrials.gov/ct2/show/NCT01763164 (first received 4 January 2013).
NCT01909453 {published data only}
-
- NCT01909453. Study comparing combination of LGX818 plus MEK162 versus vemurafenib and LGX818 monotherapy in BRAF mutant melanoma (COLUMBUS). clinicaltrials.gov/ct2/show/NCT01909453 (first received 24 July 2013).
NCT01940809 {published data only}
-
- NCT01940809. Ipilimumab with or without dabrafenib, trametinib, and/or nivolumab in treating patients with melanoma that is metastatic or cannot be removed by surgery. clinicaltrials.gov/ct2/show/NCT01940809 (first received 9 September 2013).
NCT01943422 {published data only}
-
- NCT01943422. Safety and efficacy study of vemurafenib and high-dose interferon alfa-2b in melanoma (12-107). clinicaltrials.gov/ct2/show/NCT01943422 (first received 27 August 2013).
NCT02130466 {published data only}
-
- NCT02130466. A study of the safety and efficacy of pembrolizumab (MK-3475) in combination with trametinib and dabrafenib in participants with advanced melanoma (MK-3475-022/KEYNOTE-022). clinicaltrials.gov/ct2/show/NCT02130466 (first received 1 May 2014).
NCT02224781 {published data only}
-
- NCT02224781. Dabrafenib and trametinib followed by ipilimumab and nivolumab or ipilimumab and nivolumab followed by dabrafenib and trametinib in treating patients with stage III-IV BRAFV600 melanoma. clinicaltrials.gov/ct2/show/NCT02224781 (first received 22 August 2014).
NCT02278887 {published data only}
-
- NCT02278887. Study comparing TIL to standard ipilimumab in patients with metastatic melanoma (TIL). clinicaltrials.gov/ct2/show/NCT02278887 (first received 3 June 2014).
NCT02339571 {published data only}
-
- NCT02339571. Nivolumab and ipilimumab with or without sargramostim in treating patients with stage III-IV melanoma that cannot be removed by surgery. clinicaltrials.gov/ct2/show/NCT02339571 (first received 12 January 2015).
NCT02388906 {published data only}
-
- NCT02388906. Efficacy study of nivolumab compared to ipilimumab in prevention of recurrence of melanoma after complete resection of stage IIIb/c or stage IV melanoma (CheckMate 238). clinicaltrials.gov/ct2/show/NCT02388906 (first received 10 March 2015).
NCT02416232 {published data only}
-
- NCT02416232. Access study of trametinib for subjects with advanced unresectable (Stage IIIc) or distant metastatic (Stage IV) BRAF V600E/K mutation positive cutaneous melanoma. clinicaltrials.gov/ct2/show/NCT02416232 (first received 9 April 2015).
NCT02460068 {published data only}
-
- NCT02460068. A study of fotemustine(FTM) vs FTM and ipilimumab (IPI) or IPI and nivolumab in melanoma brain metastasis (NIBIT-M2). clinicaltrials.gov/ct2/show/NCT02460068 (first received 22 May 2015).
NCT02506153 {published data only}
-
- NCT02506153. High-dose recombinant interferon alfa-2B, ipilimumab, or pembrolizumab in treating patients with stage III-IV high risk melanoma that has been removed by surgery. clinicaltrials.gov/ct2/show/NCT02506153 (first received 22 July 2015).
NCT02599402 {published data only}
-
- NCT02599402. Nivolumab combined with ipilimumab followed by nivolumab monotherapy as first-line treatment for patients with advanced melanoma (CheckMate 401). clinicaltrials.gov/ct2/show/NCT02599402 (first received 5 November 2015).
NCT02625337 {published data only}
-
- NCT02625337. Study comparing pembrolizumab with dual MAPK pathway inhibition plus pembrolizumab in melanoma patients (IMPemBra). clinicaltrials.gov/ct2/show/NCT02625337 (first received 1 December 2015).
NCT02714218 {published data only}
-
- NCT02714218. A study of two different dose combinations of nivolumab in combination with ipilimumab in subjects with previously untreated, unresectable or metastatic melanoma. clinicaltrials.gov/ct2/show/NCT02714218 (first received 16 March 2016).
NCT02752074 {published data only}
-
- NCT02752074. A phase 3 study of pembrolizumab + epacadostat or placebo in subjects with unresectable or metastatic melanoma (Keynote-252 / ECHO-301). clinicaltrials.gov/ct2/show/NCT02752074 (first received 22 April 2016).
NCT02821013 {published data only}
-
- NCT02821013. Duration of anti-PD-1 therapy in metastatic melanoma (STOP-GAP). clinicaltrials.gov/ct2/show/NCT02821013 (first received 29 June 2016).
Additional references
Altman 1999
Altman 2002
Arkenau 2011
Ashour 2017
Australian and New Zealand 2008
-
- Australian Cancer Network Melanoma Guidelines Revision Working Party. Clinical Practice Guidelines for the Management of Melanoma in Australia and New Zealand (CP111). Wellington 2008. [www.nhmrc.gov.au/_files_nhmrc/publications/attachments/cp111.pdf]
Bajetta 2006
-
- Bajetta E, Del Vecchio M, Nova P, Fusi A, Daponte A, Sertoli MR, et al. Multicenter phase III randomized trial of polychemotherapy (CVD regimen) versus the same chemotherapy (CT) plus subcutaneous interleukin-2 and interferon-alpha2b in metastatic melanoma. Annals of Oncology 2006;17(4):571-7. [PMID: ] - PubMed
Balch 2001
-
- Balch CM, Soong SJ, Gershenwald JE, Thompson JF, Reintgen DS, Cascinelli N, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. Journal of Clinical Oncology 2001;19(16):3622-34. [PMID: ] - PubMed
Balch 2009
Belardelli 2002
-
- Belardelli F, Ferrantini M, Proietti E, Kirkwood JM. Interferon-alpha in tumor immunity and immunotherapy. Cytokine & Growth Factor Reviews 2002;13(2):119-34. [MEDLINE: ] - PubMed
Beusterien 2003
-
- Beusterien KM, Ackerman SJ, Plante K, Glaspy J, Naredi P, Wood D, et al. The health-related quality-of-life impact of histamine dihydrochloride plus interleukin-2 compared with interleukin-2 alone in patients with metastatic melanoma. Supportive Care in Cancer 2003;11(5):304-12. [PMID: ] - PubMed
Borenstein 2009
-
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Multiple comparisons within a study. In: Introduction to Meta-analysis. 1st edition. Wiley-Blackwell, 2009:239-242.
Brundage 1993
-
- Brundage MD, Pater JL, Zee B. Assessing the reliability of two toxicity scales: implications for interpreting toxicity data. Journal of the National Cancer Institute 1993;85(14):1138-48. [PMID: ] - PubMed
Caino 2016
Cancer Research UK 2017
-
- Cancer Research UK 2017. General cancer information. www.about-cancer.cancerresearchuk.org/about-cancer/cancer-in-general/tre... (accessed 13 December 2017).
Case 2002
-
- Case LD, Kimmick G, Paskett ED, Lohman K, Tucker R. Interpreting measures of treatment effect in cancer clinical trials. Oncologist 2002;7(3):181-7. [PMID: 12065789 ] - PubMed
Chaimani 2013
Chi 2011
-
- Chi M, Dudek AZ. Vaccine therapy for metastatic melanoma: systematic review and meta-analysis of clinical trials. Melanoma Research 2011;21(3):165-74. [MEDLINE: ] - PubMed
Chiarion‐Sileni 2003
-
- Chiarion-Sileni V, Del Bianco P, De Salvo GL, Lo Re G, Romanini A, Labianca R, et al. Quality of life evaluation in a randomised trial of chemotherapy versus bio-chemotherapy in advanced melanoma patients. European Journal of Cancer 2003;39(11):1577-85. [PMID: ] - PubMed
Cipriani 2013
-
- Cipriani A, Higgins JP, Geddes JR, Salanti G. Conceptual and technical challenges in network meta-analysis. Annals of Internal Medicine 2013;159(2):130-7. [PMID: ] - PubMed
Ciren 2016
Coates 1993
-
- Coates A, Thomson D, McLeod GR, Hersey P, Gill PG, Olver IN, et al. Prognostic value of quality of life scores in a trial of chemotherapy with or without interferon in patients with metastatic malignant melanoma. European Journal of Cancer 1993;29A(12):1731-4. [PMID: ] - PubMed
Davies 2002
-
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature 2002;417(6892):949-54. [PMID: ] - PubMed
Devji 2017
-
- Devji T, Levine O, Neupane B, Beyene J, Xie F. Systemic therapy for previously untreated advanced BRAF-mutated melanoma: a systematic review and network meta-analysis of randomized clinical trials. JAMA Oncology 2017;3(3):366-73. [PMID: ] - PubMed
Di Giacomo 2012
-
- Di Giacomo AM, Ascierto PA, Pilla L, Santinami M, Ferrucci PF, Giannarelli D, et al. Ipilimumab and fotemustine in patients with advanced melanoma (NIBIT-M1): an open-label, single-arm phase 2 trial. Lancet Oncology 2012;13(9):879-86. [PMID: ] - PubMed
Donegan 2013
-
- Donegan S, Williamson P, D'Alessandro U, Tudur Smith C. Assessing key assumptions of network meta-analysis: a review of methods. Research Synthesis Methods 2013;4(4):291-323. [PMID: ] - PubMed
Edlundh‐Rose 2006
-
- Edlundh-Rose E, Egyhazi S, Omholt K, Mansson-Brahme E, Platz A, Hansson J, et al. NRAS and BRAF mutations in melanoma tumours in relation to clinical characteristics: a study based on mutation screening by pyrosequencing. Melanoma Research 2006;16(6):471-8. [PMID: ] - PubMed
Eggermont 2009
Ferlay 2010
-
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International journal of cancer 2010;127(12):2893-917. [PMID: ] - PubMed
Flaherty 2012
Flaherty 2013
Forsea 2012
-
- Forsea AM, Del Marmol V, Vries E, Bailey EE, Geller AC. Melanoma incidence and mortality in Europe: new estimates, persistent disparities. British Journal of Dermatology 2012;167(5):1124-30. [PMID: ] - PubMed
Garbe 2011
Gentile 2017
-
- Gentile C, Martorana A, Lauria A, Bonsignore R. Kinase inhibitors in multitargeted cancer therapy. Current Medicinal Chemistry 2017;24(16):1671-86. [PMID: ] - PubMed
Gorry 2018
Grob 2014
-
- Grob JJ, Amonkar MM, Martin-Algarra S, Demidov LV, Goodman V, Grotzinger K, et al. Patient perception of the benefit of a BRAF inhibitor in metastatic melanoma: quality-of-life analyses of the BREAK-3 study comparing dabrafenib with dacarbazine. Annals of Oncology 2014;25(7):1428-36. [PMID: ] - PubMed
Grob 2015
-
- Grob JJ, Amonkar MM, Karaszewska B, Schachter J, Dummer R, Mackiewicz A, et al. Comparison of dabrafenib and trametinib combination therapy with vemurafenib monotherapy on health-related quality of life in patients with unresectable or metastatic cutaneous BRAF Val600-mutation-positive melanoma (COMBI-v): results of a phase 3, open-label, randomised trial. Lancet Oncology 2015;16(13):1389-98. [PMID: ] - PubMed
Guo 2011
-
- Guo J, Si L, Kong Y, Flaherty KT, Xu X, Zhu Y, et al. Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. Journal of Clinical Oncology 2011;29(21):2904-9. [PMID: ] - PubMed
Guyatt 2008
Gyorki 2013
Hamid 2013
Hamm 2008
-
- Hamm C, Verma S, Petrella T, Bak K, Charette M, Melanoma Disease Site Group of Cancer Care Ontario's Program in Evidence-based Care. Biochemotherapy for the treatment of metastatic malignant melanoma: a systematic review. Cancer Treatment Reviews 2008;34(2):145-56. [PMID: ] - PubMed
Hauschild 2009
-
- Hauschild A, Agarwala SS, Trefzer U, Hogg D, Robert C, Hersey P, et al. Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. Journal of Clinical Oncology 2009;27(17):2823-30. [PMID: ] - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]. The Cochrane Collaboration 2011. Available from www.cochrane-handbook.org.
Higgins 2012
Hillner 2000
-
- Hillner BE, Agarwala S, Middleton MR. Post hoc economic analysis of temozolomide versus dacarbazine in the treatment of advanced metastatic melanoma. Journal of Clinical Oncology 2000;18(7):1474-80. [PMID: ] - PubMed
Hodi 2010
Hong 2013
-
- Hong H, Carlin BP, Shamliyan TA, Wyman JF, Ramakrishnan R, Sainfort F, et al. Comparing Bayesian and frequentist approaches for multiple outcome mixed treatment comparisons. Medical Decision Making 2013;33(5):702-14. [PMID: ] - PubMed
Huncharek 2001
-
- Huncharek M, Caubet JF, McGarry R. Single-agent DTIC versus combination chemotherapy with or without immunotherapy in metastatic melanoma: a meta-analysis of 3273 patients from 20 randomized trials. Melanoma Research 2001;11(1):75-81. [PMID: ] - PubMed
Ives 2007
-
- Ives NJ, Stowe RL, Lorigan P, Wheatley K. Chemotherapy compared with biochemotherapy for the treatment of metastatic melanoma: a meta-analysis of 18 trials involving 2,621 patients. Journal of Clinical Oncology 2007;25(34):5426-34. [PMID: ] - PubMed
Jager 2015
-
- Jager NG, Linn SC, Schellens JH, Beijnen JH. Tailored tamoxifen treatment for breast cancer patients: a perspective. Clin Breast Cancer 2015;15(4):241-4. [PMID: ] - PubMed
Jayson 2016
-
- Jayson GC, Kerbel R, Ellis LM, Harris AL. Antiangiogenic therapy in oncology: current status and future directions. Lancet 2016;388(10043):518-29. [PMID: ] - PubMed
Keilholz 2002
-
- Keilholz U, Gore ME. Biochemotherapy for advanced melanoma. Seminars in Oncology 2002;29(5):456-61. [PMID: ] - PubMed
Kiebert 2003
-
- Kiebert GM, Jonas DL, Middleton MR. Health-related quality of life in patients with advanced metastatic melanoma: results of a randomized phase III study comparing temozolomide with dacarbazine. Cancer Investigation 2003;21(6):821-9. [PMID: ] - PubMed
Kirkwood 2008
-
- Kirkwood JM, Tarhini AA, Panelli MC, Moschos SJ, Zarour HM, Butterfield LH, et al. Next generation of immunotherapy for melanoma. Journal of Clinical Oncology 2008;26(20):3445-55. [PMID: ] - PubMed
Kirkwood 2012
Kirkwood 2012a
Klein 2013
-
- Klein O, Clements A, Menzies AM, O'Toole S, Kefford RF, Long GV. BRAF inhibitor activity in V600R metastatic melanoma. European Journal of Cancer 2013;49(5):1073-9. [PMID: ] - PubMed
La Porta 2007
-
- La Porta CA. Drug resistance in melanoma: new perspectives. Current Medicinal Chemistry 2007;14(4):387-91. [PMID: ] - PubMed
La Porta 2009
-
- La Porta CA. Mechanism of drug sensitivity and resistance in melanoma. Current Cancer Drug Targets 2009;9(3):391-7. [PMID: ] - PubMed
Little 2012
-
- Little EG, Eide MJ. Update on the current state of melanoma incidence. Dermatologic Clinics 2012;30(3):355-61. [MEDLINE: ] - PubMed
Long 2011
-
- Long GV, Menzies AM, Nagrial AM, Haydu LE, Hamilton AL, Mann GJ, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. Journal of Clinical Oncology 2011;29(10):1239-46. [PMID: ] - PubMed
Long 2012
-
- Long GV, Trefzer U, Davies MA, Kefford RF, Ascierto PA, Chapman PB, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncology 2012;13(11):1087-95. [PMID: ] - PubMed
Long 2012a
-
- Long GV, Trefzer U, Davies MA, Kefford RF, Ascierto PA, Chapman PB, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncology 2012;13(11):1087-95. [PMID: ] - PubMed
Margolin 2012
-
- Margolin K, Ernstoff MS, Hamid O, Lawrence D, McDermott D, Puzanov I, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncology 2012;13(5):459-65. [PMID: ] - PubMed
McKinnon 2005
Meckbach 2014
Melanoma Institute Australia 2017
-
- Melanoma Institute Australia. Glossary. www.melanoma.org.au/understanding-melanoma/glossary/braf/ (accessed 13 December 2017).
Menzies 2013
-
- Menzies AM, Kefford RF, Long GV. Paradoxical oncogenesis: are all BRAF inhibitors equal? Pigment Cell & Melanoma Research 2013;26(5):611-5. [PMID: ] - PubMed
Mills 2013
-
- Mills EJ, Thorlund K, Ioannidis JP. Demystifying trial networks and network meta-analysis. BMJ (Clinical research ed.) 2013;346:f2914. [PMID: ] - PubMed
Mocellin 2003
-
- Mocellin S, Rossi CR, Nitti D, Lise M, Marincola FM. Dissecting tumor responsiveness to immunotherapy: the experience of peptide-based melanoma vaccines. Biochim Biophys Acta. 2003 Dec 5;1653(2):61-71 2003;1653:61-71. - PubMed
Mocellin 2004
-
- Mocellin S, Mandruzzato S, Bronte V, Lise M, Nitti D. Part I: Vaccines for solid tumours. Lancet Oncology 2004;5(11):681-9. [MEDLINE: ] - PubMed
Mocellin 2005
-
- Mocellin S. Cancer vaccines: the challenge of developing an ideal tumor killing system. Frontiers in Bioscience 2005;10:2285-305. [MEDLINE: ] - PubMed
Mocellin 2008
-
- Mocellin S, Nitti D. Therapeutics targeting tumor immune escape: towards the development of new generation anticancer vaccines. Medicinal Research Reviews 2008;28(3):413-44. [MEDLINE: ] - PubMed
Mocellin 2009
-
- Mocellin S, Pilati P, Nitti D. Peptide-based anticancer vaccines: recent advances and future perspectives. Curr Med Chem 2009;16:4779-96. - PubMed
Mocellin 2010
-
- Mocellin S, Pasquali S, Rossi CR, Nitti D. Interferon alpha adjuvant therapy in patients with high-risk melanoma: a systematic review and meta-analysis. Journal of the National Cancer Institute 2010;102(7):493-501. [PMID: ] - PubMed
Mocellin 2010a
Mocellin 2011
-
- Mocellin S, Pasquali S, Nitti D. The impact of surgery on survival of patients with cutaneous melanoma: revisiting the role of primary tumor excision margins. Annals of Surgery 2011;253(2):238-43. [PMID: ] - PubMed
Mocellin 2013
Mocellin 2013a
-
- Mocellin S, Benna C, Pilati P. Coinhibitory molecules in cancer biology and therapy. Cytokine & Growth Factor Reviews 2013;24(2):147-61. [PMID: ] - PubMed
Mocellin 2013b
National Toxicology Program 2011
-
- National Toxicology Program. Dacarbazine. Report on carcinogens : carcinogen profiles / U.S. Dept. of Health and Human Services, Public Health Service, National Toxicology Program 2011;12:127-8. [PMID: ] - PubMed
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815-34. [PMID: ] - PubMed
Pasquali 2010
-
- Pasquali S, Mocellin S. The anticancer face of interferon alpha (IFN-alpha): from biology to clinical results, with a focus on melanoma. Current Medicinal Chemistry 2010;17(29):3327-36. [MEDLINE: ] - PubMed
Pasquali 2013
-
- Pasquali S, Haydu LE, Scolyer RA, Winstanley JB, Spillane AJ, Quinn MJ, et al. The importance of adequate primary tumor excision margins and sentinel node biopsy in achieving optimal locoregional control for patients with thick primary melanomas. Annals of Surgery 2013;258(1):152-7. [PMID: ] - PubMed
Pasquali 2014
Pasquali 2017
-
- Pasquali S, Chiarion-Sileni V, Rossi CR, Mocellin S. Immune checkpoint inhibitors and targeted therapies for metastatic melanoma: A network meta-analysis. Cancer Treatment Reviews 2017;54:34-42. [PMID: ] - PubMed
Revicki 2012
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ridolfi 2002
-
- Ridolfi R, Chiarion-Sileni V, Guida M, Romanini A, Labianca R, Freschi A, et al. Cisplatin, dacarbazine with or without subcutaneous interleukin-2, and interferon alpha-2b in advanced melanoma outpatients: results from an Italian multicenter phase III randomized clinical trial. Journal of Clinical Oncology 2002;20(6):1600-7. [PMID: ] - PubMed
Salanti 2011
-
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. Journal of Clinical Epidemiology 2011;64(2):163-71. [PMID: ] - PubMed
Salanti 2014
Sasse 2007
Schadendorf 2009
Schadendorf 2014
-
- Schadendorf D, Amonkar MM, Milhem M, Grotzinger K, Demidov LV, Rutkowski P, et al. Functional and symptom impact of trametinib versus chemotherapy in BRAF V600E advanced or metastatic melanoma: quality-of-life analyses of the METRIC study. Annals of Oncology 2014;25(3):700-6. [PMID: ] - PMC - PubMed
Schadendorf 2015
-
- Schadendorf D, Amonkar MM, Stroyakovskiy D, Levchenko E, Gogas H, Braud F, et al. Health-related quality of life impact in a randomised phase III study of the combination of dabrafenib and trametinib versus dabrafenib monotherapy in patients with BRAF V600 metastatic melanoma. European Journal of Cancer 2015;51(7):833-40. [PMID: ] - PubMed
Schulz 2010
Schwartzentruber 2011
Scolyer 2011
Serrone 1999
-
- Serrone L, Hersey P. The chemoresistance of human malignant melanoma: an update. Melanoma Research 1999;9(1):51-8. [MEDLINE: ] - PubMed
Sherrill 2013
Siegel 2012
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: a Cancer Journal for Clinicians 2012;62(1):10-29. [PMID: ] - PubMed
Sladden 2009
Sosman 2012
Stata 2017 [Computer program]
-
- Stata. Version 11.2. College Station, TX, USA: StataCorp, 2017. Available at www.stata.com.
Stevens 2006
-
- Stevens G, McKay MJ. Dispelling the myths surrounding radiotherapy for treatment of cutaneous melanoma. Lancet Oncology 2006;7(7):575-83. [PMID: ] - PubMed
Tarhini 2005
-
- Tarhini AA, Agarwala SS. Interleukin-2 for the treatment of melanoma. Current opinion in Investigational Drugs 2005;6(12):1234-9. [PMID: ] - PubMed
Testori 2009
Therasse 2002
-
- Therasse P. Measuring the clinical response. What does it mean? European Journal of Cancer 2002;38(14):1817-23. [PMID: ] - PubMed
Thompson 2009
-
- Thompson JF, Scolyer RA, Kefford RF. Cutaneous melanoma in the era of molecular profiling. Lancet 2009;374(9687):362-5. [PMID: ] - PubMed
Tierney 2007
Wevers 2013
-
- Wevers KP, Hoekstra HJ. Stage IV melanoma: completely resectable patients are scarce. Annals of Surgical Oncology 2013;20(7):2352-6. [PMID: ] - PubMed
White 2011
-
- White IR. Multivariate random-effects meta-regression: Updates to mvmeta. Stata Journal 2011;11(2):255-70. [www.stata-journal.com/sj11-2.html]
White 2012
White 2015
-
- White I. Network meta-analysis. Stata Journal 2015;15(4):951-85.
Wise 2016
-
- Wise J. NICE approves immunotherapy combination for advanced melanoma. BMJ 2016;353:i3421. [PMID: ] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

