Increased central common drive to ankle plantar flexor and dorsiflexor muscles during visually guided gait
- PMID: 29405634
- PMCID: PMC5800295
- DOI: 10.14814/phy2.13598
Increased central common drive to ankle plantar flexor and dorsiflexor muscles during visually guided gait
Abstract
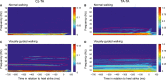
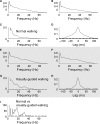
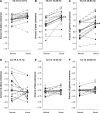
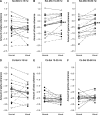
When we walk in a challenging environment, we use visual information to modify our gait and place our feet carefully on the ground. Here, we explored how central common drive to ankle muscles changes in relation to visually guided foot placement. Sixteen healthy adults aged 23 ± 5 years participated in the study. Electromyography (EMG) from the Soleus (Sol), medial Gastrocnemius (MG), and the distal and proximal ends of the Tibialis anterior (TA) muscles and electroencephalography (EEG) from Cz were recorded while subjects walked on a motorized treadmill. A visually guided walking task, where subjects received visual feedback of their foot placement on a screen in real-time and were required to place their feet within narrow preset target areas, was compared to normal walking. There was a significant increase in the central common drive estimated by TA-TA and Sol-MG EMG-EMG coherence in beta and gamma frequencies during the visually guided walking compared to normal walking. EEG-TA EMG coherence also increased, but the group average did not reach statistical significance. The results indicate that the corticospinal tract is involved in modifying gait when visually guided placement of the foot is required. These findings are important for our basic understanding of the central control of human bipedal gait and for the design of rehabilitation interventions for gait function following central motor lesions.
Keywords: EMG; coherence; locomotion; visually guided walking.
© 2018 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Figures

References
-
- Amjad, A. M. , Halliday D. M., Rosenberg J. R., and Conway B. A.. 1997. An extended difference of coherence test for comparing and combining several independent coherence estimates: theory and application to the study of motor units and physiological tremor. J. Neurosci. Methods 73:69–79. - PubMed
-
- Amos, A. , Armstrong D. M., and Marple‐Horvat D. E.. 1990. Changes in the discharge patterns of motor cortical neurones associated with volitional changes in stepping in the cat. Neurosci. Lett. 109:107–112. - PubMed
-
- Barthelemy, D. , Willerslev‐Olsen M., Lundell H., Conway B. A., Knudsen H., Biering‐Sørensen F., et al. 2010. Impaired transmission in the corticospinal tract and gait disability in spinal cord injured persons. J. Neurophysiol. 104:1167–1176. - PubMed
-
- Christensen, L. O. , Johannsen P., Sinkjaer T., Petersen N., Pyndt H. S., and Nielsen J. B.. 2000. Cerebral activation during bicycle movements in man. Exp. Brain Res. 135:66–72. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

