Liquid biopsy and its role in an advanced clinical trial for lung cancer
- PMID: 29405770
- PMCID: PMC5813874
- DOI: 10.1177/1535370217750087
Liquid biopsy and its role in an advanced clinical trial for lung cancer
Abstract
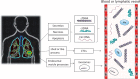
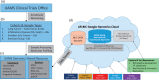
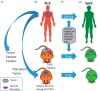
Liquid biopsy methodologies, for the purpose of plasma genotyping of cell-free DNA (cfDNA) of solid tumors, are a new class of novel molecular assays. Such assays are rapidly entering the clinical sphere of research-based monitoring in translational oncology, especially for thoracic malignancies. Potential applications for these blood-based cfDNA assays include: (i) initial diagnosis, (ii) response to therapy and follow-up, (iii) tumor evolution, and (iv) minimal residual disease evaluation. Precision medicine will benefit from cutting-edge molecular diagnostics, especially regarding treatment decisions in the adjuvant setting, where avoiding over-treatment and unnecessary toxicity are paramount. The use of innovative genetic analysis techniques on individual patient tumor samples is being pursued in several advanced clinical trials. Rather than using a categorical treatment plan, the next critical step of therapeutic decision making is providing the "right" cancer therapy for an individual patient, including correct dose and timeframe based on the molecular analysis of the tumor in question. Per the 21st Century Cures Act, innovative clinical trials are integral for biomarker and drug development. This will include advanced clinical trials utilizing: (i) innovative assays, (ii) molecular profiling with cutting-edge bioinformatics, and (iii) clinically relevant animal or tissue models. In this paper, a mini-review addresses state-of-the-art liquid biopsy approaches. Additionally, an on-going advanced clinical trial for lung cancer with novelty through synergizing liquid biopsies, co-clinical trials, and advanced bioinformatics is also presented. Impact statement Liquid biopsy technology is providing a new source for cancer biomarkers, and adds new dimensions in advanced clinical trials. Utilizing a non-invasive routine blood draw, the liquid biopsy provides abilities to address perplexing issues of tumor tissue heterogeneity by identifying mutations in both primary and metastatic lesions. Regarding the assessment of response to cancer therapy, the liquid biopsy is not ready to replace medical imaging, but adds critical new information; for instance, through a temporal assessment of quantitative circulating tumor DNA (ctDNA) assay results, and importantly, the ability to monitor for signs of resistance, via emerging clones. Adjuvant therapy may soon be considered based on a quantitative cfDNA assay. As sensitivity and specificity of the technology continue to progress, cancer screening and prevention will improve and save countless lives by finding the cancer early, so that a routine surgery may be all that is required for a definitive cure.
Keywords: Bioinformatics; biomarkers; cancer; genomics; liquid biopsy; precision medicine.
Figures

Similar articles
-
Circulating cell-free nucleic acids and platelets as a liquid biopsy in the provision of personalized therapy for lung cancer patients.Lung Cancer. 2017 May;107:100-107. doi: 10.1016/j.lungcan.2016.04.026. Epub 2016 May 4. Lung Cancer. 2017. PMID: 27180141 Review.
-
Unlocking the future of cancer diagnosis - promises and challenges of ctDNA-based liquid biopsies in non-small cell lung cancer.Transl Res. 2024 Oct;272:41-53. doi: 10.1016/j.trsl.2024.05.014. Epub 2024 Jun 3. Transl Res. 2024. PMID: 38838851 Review.
-
Current and future applications of liquid biopsy in nonsmall cell lung cancer from early to advanced stages.Eur Respir Rev. 2020 Feb 12;29(155):190052. doi: 10.1183/16000617.0052-2019. Print 2020 Mar 31. Eur Respir Rev. 2020. PMID: 32051167 Free PMC article. Review.
-
Liquid Biopsy Approaches for Cancer Characterization, Residual Disease Detection, and Therapy Monitoring.Am Soc Clin Oncol Educ Book. 2025 Jun;45(3):e481114. doi: 10.1200/EDBK-25-481114. Epub 2025 Apr 30. Am Soc Clin Oncol Educ Book. 2025. PMID: 40305739 Review.
-
Cell-free DNA and next-generation sequencing in the service of personalized medicine for lung cancer.Oncotarget. 2016 Oct 25;7(43):71013-71035. doi: 10.18632/oncotarget.11717. Oncotarget. 2016. PMID: 27589834 Free PMC article. Review.
Cited by
-
Genetic Markers in Lung Cancer Diagnosis: A Review.Int J Mol Sci. 2020 Jun 27;21(13):4569. doi: 10.3390/ijms21134569. Int J Mol Sci. 2020. PMID: 32604993 Free PMC article. Review.
-
Applications for Circulating Cell-Free DNA in Oral Squamous Cell Carcinoma: A Non-Invasive Approach for Detecting Structural Variants, Fusions, and Oncoviruses.Cancers (Basel). 2025 Jun 6;17(12):1901. doi: 10.3390/cancers17121901. Cancers (Basel). 2025. PMID: 40563552 Free PMC article.
-
The Role of Tissue and Liquid Biopsy in the Clinical Management of Adult Lung Cancer Patients in King Abdul-Aziz Medical City in Riyadh, Saudi Arabia.Cureus. 2022 Jan 3;14(1):e20914. doi: 10.7759/cureus.20914. eCollection 2022 Jan. Cureus. 2022. PMID: 35004079 Free PMC article.
-
Extracellular Vesicle Molecular Signatures Characterize Metastatic Dynamicity in Ovarian Cancer.Front Oncol. 2021 Nov 18;11:718408. doi: 10.3389/fonc.2021.718408. eCollection 2021. Front Oncol. 2021. PMID: 34868914 Free PMC article.
-
Surveillance of cfDNA Hot Spot Mutations in NSCLC Patients during Disease Progression.Int J Mol Sci. 2023 Apr 9;24(8):6958. doi: 10.3390/ijms24086958. Int J Mol Sci. 2023. PMID: 37108122 Free PMC article.
References
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646–74 - PubMed
-
- Lih C-J, Takebe N. Considerations of developing an NGS assay for clinical applications in precision oncology: the NCI-MATCH NGS assay experience. Curr Probl Cancer 2017; 41:201–11 - PubMed
-
- NCI-MATCH Trial (Molecular Analysis for Therapy Choice), Goals of NCI MATCH, www.cancer.gov/about-cancer/treatment/clinical-trials/nci-supported/nci-... (accessed 8 December 2017)
-
- Hirsch MS, Günthard HF, Schapiro JM, Vézinet FB, Clotet B, Hammer SM, Johnson VA, Kuritzkes DR, Mellors JW, Pillay D. Antiretroviral drug resistance testing in adult HIV-1 infection: 2008 recommendations of an International AIDS Society-USA panel. Clin Infect Dis 2008; 47:266–85 - PubMed
-
- Siravegna G, Marsoni S, Siena S, Bardelli A. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol 2017; 14:531–48 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

