ISCU(M108I) and ISCU(D39V) Differ from Wild-Type ISCU in Their Failure To Form Cysteine Desulfurase Complexes Containing Both Frataxin and Ferredoxin
- PMID: 29406711
- PMCID: PMC5842376
- DOI: 10.1021/acs.biochem.7b01234
ISCU(M108I) and ISCU(D39V) Differ from Wild-Type ISCU in Their Failure To Form Cysteine Desulfurase Complexes Containing Both Frataxin and Ferredoxin
Abstract
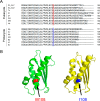
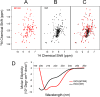
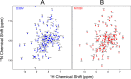
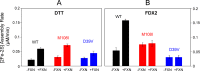
Whereas iron-sulfur (Fe-S) cluster assembly on the wild-type scaffold protein ISCU, as catalyzed by the human cysteine desulfurase complex (NIA)2, exhibits a requirement for frataxin (FXN), in yeast, ISCU variant M108I has been shown to bypass the FXN requirement. Wild-type ISCU populates two interconverting conformational states: one structured and one dynamically disordered. We show here that variants ISCU(M108I) and ISCU(D39V) of human ISCU populate only the structured state. We have compared the properties of ISCU, ISCU(M108I), and ISCU(D39V), with and without FXN, in both the cysteine desulfurase step of Fe-S cluster assembly and the overall Fe-S cluster assembly reaction catalyzed by (NIA)2. In the cysteine desulfurase step with dithiothreitol (DTT) as the reductant, FXN was found to stimulate cysteine desulfurase activity with both the wild-type and structured variants, although the effect was less prominent with ISCU(D39V) than with the wild-type or ISCU(M108I). In overall Fe-S cluster assembly, frataxin was found to stimulate cluster assembly with both the wild-type and structured variants when the reductant was DTT; however, with the physiological reductant, reduced ferredoxin 2 (rdFDX2), FXN stimulated the reaction with wild-type ISCU but not with either ISCU(M108I) or ISCU(D39V). Nuclear magnetic resonance titration experiments revealed that wild-type ISCU, FXN, and rdFDX2 all bind to (NIA)2. However, when ISCU was replaced by the fully structured variant ISCU(M108I), the addition of rdFDX2 to the [NIA-ISCU(M108I)-FXN]2 complex led to the release of FXN. Thus, the displacement of FXN by rdFDX2 explains the failure of FXN to stimulate Fe-S cluster assembly on ISCU(M108I).
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Interactions of iron-bound frataxin with ISCU and ferredoxin on the cysteine desulfurase complex leading to Fe-S cluster assembly.J Inorg Biochem. 2018 Jun;183:107-116. doi: 10.1016/j.jinorgbio.2018.03.007. Epub 2018 Mar 15. J Inorg Biochem. 2018. PMID: 29576242 Free PMC article.
-
Zinc(II) binding on human wild-type ISCU and Met140 variants modulates NFS1 desulfurase activity.Biochimie. 2018 Sep;152:211-218. doi: 10.1016/j.biochi.2018.07.012. Epub 2018 Jul 20. Biochimie. 2018. PMID: 30031876 Free PMC article.
-
Human Mitochondrial Ferredoxin 1 (FDX1) and Ferredoxin 2 (FDX2) Both Bind Cysteine Desulfurase and Donate Electrons for Iron-Sulfur Cluster Biosynthesis.Biochemistry. 2017 Jan 24;56(3):487-499. doi: 10.1021/acs.biochem.6b00447. Epub 2017 Jan 11. Biochemistry. 2017. PMID: 28001042 Free PMC article.
-
Molecular Details of the Frataxin-Scaffold Interaction during Mitochondrial Fe-S Cluster Assembly.Int J Mol Sci. 2021 Jun 2;22(11):6006. doi: 10.3390/ijms22116006. Int J Mol Sci. 2021. PMID: 34199378 Free PMC article. Review.
-
Metamorphic protein IscU alternates conformations in the course of its role as the scaffold protein for iron-sulfur cluster biosynthesis and delivery.FEBS Lett. 2013 Apr 17;587(8):1172-9. doi: 10.1016/j.febslet.2013.01.003. Epub 2013 Jan 16. FEBS Lett. 2013. PMID: 23333622 Free PMC article. Review.
Cited by
-
Interactions of iron-bound frataxin with ISCU and ferredoxin on the cysteine desulfurase complex leading to Fe-S cluster assembly.J Inorg Biochem. 2018 Jun;183:107-116. doi: 10.1016/j.jinorgbio.2018.03.007. Epub 2018 Mar 15. J Inorg Biochem. 2018. PMID: 29576242 Free PMC article.
-
Mammalian mitochondrial iron-sulfur cluster biogenesis and transfer and related human diseases.Biophys Rep. 2021 Apr 30;7(2):127-141. doi: 10.52601/bpr.2021.200038. Biophys Rep. 2021. PMID: 37288145 Free PMC article.
-
Hypoxia Rescues Frataxin Loss by Restoring Iron Sulfur Cluster Biogenesis.Cell. 2019 May 30;177(6):1507-1521.e16. doi: 10.1016/j.cell.2019.03.045. Epub 2019 Apr 25. Cell. 2019. PMID: 31031004 Free PMC article.
-
NMR as a Tool to Investigate the Processes of Mitochondrial and Cytosolic Iron-Sulfur Cluster Biosynthesis.Molecules. 2018 Aug 31;23(9):2213. doi: 10.3390/molecules23092213. Molecules. 2018. PMID: 30200358 Free PMC article. Review.
-
Characteristics of the Isu1 C-terminus in relation to [2Fe-2S] cluster assembly and ISCU Myopathy.J Biol Inorg Chem. 2022 Dec;27(8):759-773. doi: 10.1007/s00775-022-01964-1. Epub 2022 Oct 30. J Biol Inorg Chem. 2022. PMID: 36309885
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

