Regulation of fibrosis in muscular dystrophy
- PMID: 29408413
- PMCID: PMC6519730
- DOI: 10.1016/j.matbio.2018.01.014
Regulation of fibrosis in muscular dystrophy
Abstract
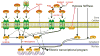
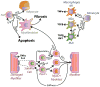
The production of force and power are inherent properties of skeletal muscle, and regulated by contractile proteins within muscle fibers. However, skeletal muscle integrity and function also require strong connections between muscle fibers and their extracellular matrix (ECM). A well-organized and pliant ECM is integral to muscle function and the ability for many different cell populations to efficiently migrate through ECM is critical during growth and regeneration. For many neuromuscular diseases, genetic mutations cause disruption of these cytoskeletal-ECM connections, resulting in muscle fragility and chronic injury. Ultimately, these changes shift the balance from myogenic pathways toward fibrogenic pathways, culminating in the loss of muscle fibers and their replacement with fatty-fibrotic matrix. Hence a common pathological hallmark of muscular dystrophy is prominent fibrosis. This review will cover the salient features of muscular dystrophy pathogenesis, highlight the signals and cells that are important for myogenic and fibrogenic actions, and discuss how fibrosis alters the ECM of skeletal muscle, and the consequences of fibrosis in developing therapies.
Keywords: Mechanosensing; Muscle regeneration; Satellite cells; Transforming growth factor beta.
Copyright © 2018. Published by Elsevier B.V.
Figures

References
-
- Romitti PA, Zhu Y, Puzhankara S, James KA, Nabukera SK, Zamba GK, Ciafaloni E, Cunniff C, Druschel CM, Mathews KD, Matthews DJ, Meaney FJ, Andrews JG, Conway KM, Fox DJ, Street N, Adams MM, Bolen J M. STARnet. Prevalence of Duchenne and Becker muscular dystrophies in the United States. Pediatrics. 2015;135:513–521. - PMC - PubMed
-
- Hoffman EP, Brown RH, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. - PubMed
-
- Theadom A, Rodrigues M, Roxburgh R, Balalla S, Higgins C, Bhattacharjee R, Jones K, Krishnamurthi R, Feigin V. Prevalence of muscular dystrophies: a systematic literature review. Neuroepidemiology. 2014;43:259–268. - PubMed
-
- Mercuri E, Muntoni F. Muscular dystrophies. Lancet. 2013;381:845–860. - PubMed
-
- Ervasti JM, Campbell KP. Membrane organization of the dystrophin-glycoprotein complex. Cell. 1991;66:1121–1131. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

