FoxO transcription factors are required for hepatic HDL cholesterol clearance
- PMID: 29408809
- PMCID: PMC5873864
- DOI: 10.1172/JCI94230
FoxO transcription factors are required for hepatic HDL cholesterol clearance
Abstract
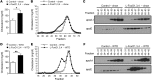
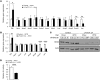
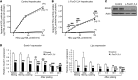
Insulin resistance and type 2 diabetes are associated with low levels of high-density lipoprotein cholesterol (HDL-C). The insulin-repressible FoxO transcription factors are potential mediators of the effect of insulin on HDL-C. FoxOs mediate a substantial portion of insulin-regulated transcription, and poor FoxO repression is thought to contribute to the excessive glucose production in diabetes. In this work, we show that mice with liver-specific triple FoxO knockout (L-FoxO1,3,4), which are known to have reduced hepatic glucose production, also have increased HDL-C. This was associated with decreased expression of the HDL-C clearance factors scavenger receptor class B type I (SR-BI) and hepatic lipase and defective selective uptake of HDL cholesteryl ester by the liver. The phenotype could be rescued by re-expression of SR-BI. These findings demonstrate that hepatic FoxOs are required for cholesterol homeostasis and HDL-mediated reverse cholesterol transport to the liver.
Keywords: Cholesterol; Insulin signaling; Lipoproteins; Metabolism.
Conflict of interest statement
Figures




Similar articles
-
Hepatic Overexpression of Endothelial Lipase Lowers High-Density Lipoprotein but Maintains Reverse Cholesterol Transport in Mice: Role of Scavenger Receptor Class B Type I/ATP-Binding Cassette Transporter A1-Dependent Pathways.Arterioscler Thromb Vasc Biol. 2018 Jul;38(7):1454-1467. doi: 10.1161/ATVBAHA.118.311056. Epub 2018 May 10. Arterioscler Thromb Vasc Biol. 2018. PMID: 29748333 Free PMC article.
-
MicroRNA-24 aggravates atherosclerosis by inhibiting selective lipid uptake from HDL cholesterol via the post-transcriptional repression of scavenger receptor class B type I.Atherosclerosis. 2018 Mar;270:57-67. doi: 10.1016/j.atherosclerosis.2018.01.045. Epub 2018 Feb 4. Atherosclerosis. 2018. PMID: 29407889
-
Enhanced cellular uptake of remnant high-density lipoprotein particles: a mechanism for high-density lipoprotein lowering in insulin resistance and hypertriglyceridemia.Circ Res. 2008 Jul 18;103(2):159-66. doi: 10.1161/CIRCRESAHA.108.178756. Epub 2008 Jun 12. Circ Res. 2008. PMID: 18556574
-
Alterations in high-density lipoprotein metabolism and reverse cholesterol transport in insulin resistance and type 2 diabetes mellitus: role of lipolytic enzymes, lecithin:cholesterol acyltransferase and lipid transfer proteins.Eur J Clin Invest. 2003 Dec;33(12):1051-69. doi: 10.1111/j.1365-2362.2003.01263.x. Eur J Clin Invest. 2003. PMID: 14636288 Review.
-
Role of lipases, lecithin:cholesterol acyltransferase and cholesteryl ester transfer protein in abnormal high density lipoprotein metabolism in insulin resistance and type 2 diabetes mellitus.Clin Lab. 2003;49(11-12):601-13. Clin Lab. 2003. PMID: 14651331 Review.
Cited by
-
Hepatic FoxOs link insulin signaling with plasma lipoprotein metabolism through an apolipoprotein M/sphingosine-1-phosphate pathway.J Clin Invest. 2022 Apr 1;132(7):e146219. doi: 10.1172/JCI146219. J Clin Invest. 2022. PMID: 35104242 Free PMC article.
-
Dysfunction of Autophagy in Adipose Tissue Macrophages Regulated via FoxO1 in Obesity-Related Severe Acute Pancreatitis.Int J Mol Sci. 2025 Jul 25;26(15):7206. doi: 10.3390/ijms26157206. Int J Mol Sci. 2025. PMID: 40806352 Free PMC article.
-
An integrative transcriptional logic model of hepatic insulin resistance.Proc Natl Acad Sci U S A. 2021 Nov 9;118(45):e2102222118. doi: 10.1073/pnas.2102222118. Proc Natl Acad Sci U S A. 2021. PMID: 34732569 Free PMC article.
-
Increased apolipoprotein C3 drives cardiovascular risk in type 1 diabetes.J Clin Invest. 2019 Jul 11;129(10):4165-4179. doi: 10.1172/JCI127308. J Clin Invest. 2019. PMID: 31295146 Free PMC article.
-
Recent advances in understanding and managing cholesterol gallstones.F1000Res. 2018 Sep 24;7:F1000 Faculty Rev-1529. doi: 10.12688/f1000research.15505.1. eCollection 2018. F1000Res. 2018. PMID: 30345010 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

