Axin phosphorylation in both Wnt-off and Wnt-on states requires the tumor suppressor APC
- PMID: 29408853
- PMCID: PMC5800574
- DOI: 10.1371/journal.pgen.1007178
Axin phosphorylation in both Wnt-off and Wnt-on states requires the tumor suppressor APC
Abstract
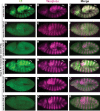
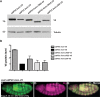
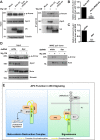
The aberrant activation of Wnt signal transduction initiates the development of 90% of colorectal cancers, the majority of which arise from inactivation of the tumor suppressor Adenomatous polyposis coli (APC). In the classical model for Wnt signaling, the primary role of APC is to act, together with the concentration-limiting scaffold protein Axin, in a "destruction complex" that directs the phosphorylation and consequent proteasomal degradation of the transcriptional activator β-catenin, thereby preventing signaling in the Wnt-off state. Following Wnt stimulation, Axin is recruited to a multiprotein "signalosome" required for pathway activation. Whereas it is well-documented that APC is essential in the destruction complex, APC's role in this complex remains elusive. Here, we demonstrate in Drosophila that Axin exists in two distinct phosphorylation states in Wnt-off and Wnt-on conditions, respectively, that underlie its roles in the destruction complex and signalosome. These two Axin phosphorylation states are catalyzed by glycogen synthase kinase 3 (GSK3), and unexpectedly, completely dependent on APC in both unstimulated and Wnt-stimulated conditions. In a major revision of the classical model, we show that APC is essential not only in the destruction complex, but also for the rapid transition in Axin that occurs after Wnt stimulation and Axin's subsequent association with the Wnt co-receptor LRP6/Arrow, one of the earliest steps in pathway activation. We propose that this novel requirement for APC in Axin regulation through phosphorylation both prevents signaling in the Wnt-off state and promotes signaling immediately following Wnt stimulation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
The Adenomatous polyposis coli tumour suppressor is essential for Axin complex assembly and function and opposes Axin's interaction with Dishevelled.Open Biol. 2011 Nov;1(3):110013. doi: 10.1098/rsob.110013. Open Biol. 2011. PMID: 22645652 Free PMC article.
-
Dual Roles for Membrane Association of Drosophila Axin in Wnt Signaling.PLoS Genet. 2016 Dec 13;12(12):e1006494. doi: 10.1371/journal.pgen.1006494. eCollection 2016 Dec. PLoS Genet. 2016. PMID: 27959917 Free PMC article.
-
Wnt/Wingless Pathway Activation Is Promoted by a Critical Threshold of Axin Maintained by the Tumor Suppressor APC and the ADP-Ribose Polymerase Tankyrase.Genetics. 2016 May;203(1):269-81. doi: 10.1534/genetics.115.183244. Epub 2016 Mar 14. Genetics. 2016. PMID: 26975665 Free PMC article.
-
Modulation of Wnt signaling by Axin and Axil.Cytokine Growth Factor Rev. 1999 Sep-Dec;10(3-4):255-65. doi: 10.1016/s1359-6101(99)00017-9. Cytokine Growth Factor Rev. 1999. PMID: 10647780 Review.
-
Toggling a conformational switch in Wnt/β-catenin signaling: regulation of Axin phosphorylation. The phosphorylation state of Axin controls its scaffold function in two Wnt pathway protein complexes.Bioessays. 2013 Dec;35(12):1063-70. doi: 10.1002/bies.201300101. Epub 2013 Sep 19. Bioessays. 2013. PMID: 24105937 Free PMC article. Review.
Cited by
-
Rotavirus-Mediated Suppression of miRNA-192 Family and miRNA-181a Activates Wnt/β-Catenin Signaling Pathway: An In Vitro Study.Viruses. 2022 Mar 9;14(3):558. doi: 10.3390/v14030558. Viruses. 2022. PMID: 35336965 Free PMC article.
-
WNT Signaling in Disease.Cells. 2019 Aug 3;8(8):826. doi: 10.3390/cells8080826. Cells. 2019. PMID: 31382613 Free PMC article. Review.
-
Wnt/Beta-Catenin Signaling Regulation and a Role for Biomolecular Condensates.Dev Cell. 2019 Feb 25;48(4):429-444. doi: 10.1016/j.devcel.2019.01.025. Dev Cell. 2019. PMID: 30782412 Free PMC article. Review.
-
FAM83B promotes cell proliferation via regulating the expression of CDK4/CDK6/CCND1 complex in laryngeal squamous cell carcinoma.Heliyon. 2024 Apr 23;10(9):e29933. doi: 10.1016/j.heliyon.2024.e29933. eCollection 2024 May 15. Heliyon. 2024. PMID: 38707417 Free PMC article.
-
When You Come to a Fork in the Road, Take It: Wnt Signaling Activates Multiple Pathways through the APC/Axin/GSK-3 Complex.Cells. 2023 Sep 12;12(18):2256. doi: 10.3390/cells12182256. Cells. 2023. PMID: 37759479 Free PMC article. Review.
References
-
- Nusse R (2008) Wnt signaling and stem cell control. Cell Res 18: 523–527. doi: 10.1038/cr.2008.47 - DOI - PubMed
-
- MacDonald BT, Tamai K, He X (2009) Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 17: 9–26. doi: 10.1016/j.devcel.2009.06.016 - DOI - PMC - PubMed
-
- Clevers H, Nusse R (2012) Wnt/beta-catenin signaling and disease. Cell 149: 1192–1205. doi: 10.1016/j.cell.2012.05.012 - DOI - PubMed
-
- Holland JD, Klaus A, Garratt AN, Birchmeier W (2013) Wnt signaling in stem and cancer stem cells. Curr Opin Cell Biol 25: 254–264. doi: 10.1016/j.ceb.2013.01.004 - DOI - PubMed
-
- Liu C, Li Y, Semenov M, Han C, Baeg GH, et al. (2002) Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108: 837–847. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

