Five year outcomes of Boston type I keratoprosthesis as primary versus secondary penetrating corneal procedure in a matched case control study
- PMID: 29408907
- PMCID: PMC5800684
- DOI: 10.1371/journal.pone.0192381
Five year outcomes of Boston type I keratoprosthesis as primary versus secondary penetrating corneal procedure in a matched case control study
Abstract
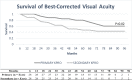
Despite improved retention and reduced complication rates paving the way for the current expansion of applications and surge in prevalence for the Boston type I Keratoprosthesis (KPro), the most frequent indication for its implantation today remains prior graft failure. The purpose of this study is to evaluate the long-term outcomes of primary KPro and compare to secondary implantation in a matched cohort study. This study included patients who underwent KPro implantation in a single center by two surgeons between July 2008 and October 2014. All eyes with KPro implantation as the primary procedure with a minimum follow up of 12 months were matched with eyes with same preoperative diagnoses that underwent secondary KPro implantation. Main outcomes included visual acuity and device retention. A total of 56 eyes were included with 28 eyes in each group. Mean follow up was 5.0 years for both groups. Twenty-nine percent (8) of the eyes in the primary group had a diagnosis of chemical or thermal injuries, 25% (7) aniridia, 18% (5) autoimmune disease, 4% (1) infectious keratitis/neurotrophic cornea, 7% (2) gelatinous corneal dystrophy, 7% (2) ectrodactyly ectodermal dysplasia/limbal stem cell deficiency, and 11% (3) uveitis/hypotony. Sixty-one percent (17) of the eyes in the primary group and 39% (11) in the secondary group maintained a final best-corrected visual acuity of 20/200 or better at a mean follow up of 5.0 years; the probability of maintaining best-corrected vision is 0.83 and 0.49 for primary and secondary groups at 5.0 years (p = 0.02). There is no statistically significant difference between groups in device retention (p = 0.22) or postoperative complication rates (p >0.05). This study demonstrates that Boston KPro implantation may be successful as a primary procedure in patients at high risk of failure with traditional penetrating keratoplasty. The device has a good long-term retention rate and visual outcomes are promising however a larger study is needed for more definitive results.
Conflict of interest statement
Figures

Similar articles
-
Glaucoma associated with Boston type I keratoprosthesis.Cornea. 2012 Feb;31(2):134-9. doi: 10.1097/ICO.0b013e31820f7a32. Cornea. 2012. PMID: 22134402 Free PMC article.
-
Visual outcomes of primary keratoprosthesis implantation in transplant-naïve eyes.PLoS One. 2024 Oct 3;19(10):e0311413. doi: 10.1371/journal.pone.0311413. eCollection 2024. PLoS One. 2024. PMID: 39361680 Free PMC article.
-
Donor Corneal Transplantation vs Boston Type 1 Keratoprosthesis in Patients with Previous Graft Failures: A Retrospective Single Center Study (An American Ophthalmological Society Thesis).Trans Am Ophthalmol Soc. 2015;113:T3. Trans Am Ophthalmol Soc. 2015. PMID: 26538773 Free PMC article.
-
The Boston Keratoprosthesis type 1 as primary penetrating corneal procedure.Br J Ophthalmol. 2015 Dec;99(12):1664-8. doi: 10.1136/bjophthalmol-2014-306161. Epub 2015 Jun 1. Br J Ophthalmol. 2015. PMID: 26034079
-
Glaucoma management in patients with penetrating keratoplasty or keratoprosthesis.Curr Opin Ophthalmol. 2023 Mar 1;34(2):95-102. doi: 10.1097/ICU.0000000000000924. Epub 2022 Nov 7. Curr Opin Ophthalmol. 2023. PMID: 36730770 Review.
Cited by
-
Application of biomaterials and nanotechnology in corneal tissue engineering.J Int Med Res. 2023 Jul;51(7):3000605231190473. doi: 10.1177/03000605231190473. J Int Med Res. 2023. PMID: 37523589 Free PMC article. Review.
-
New horizons in aniridia management: Clinical insights and therapeutic advances.Taiwan J Ophthalmol. 2023 Dec 20;13(4):467-478. doi: 10.4103/tjo.TJO-D-23-00140. eCollection 2023 Oct-Dec. Taiwan J Ophthalmol. 2023. PMID: 38249501 Free PMC article. Review.
-
Management of Congenital Aniridia-Associated Keratopathy: Long-Term Outcomes from a Tertiary Referral Center.Am J Ophthalmol. 2020 Feb;210:8-18. doi: 10.1016/j.ajo.2019.11.003. Epub 2019 Nov 12. Am J Ophthalmol. 2020. PMID: 31730836 Free PMC article.
-
Cellular therapy of corneal epithelial defect by adipose mesenchymal stem cell-derived epithelial progenitors.Stem Cell Res Ther. 2020 Jan 3;11(1):14. doi: 10.1186/s13287-019-1533-1. Stem Cell Res Ther. 2020. PMID: 31900226 Free PMC article.
-
The treatment of end-stage corneal disease: penetrating keratoplasty compared with Boston type 1 keratoprosthesis.Graefes Arch Clin Exp Ophthalmol. 2022 Sep;260(9):2781-2790. doi: 10.1007/s00417-022-05646-1. Epub 2022 Apr 6. Graefes Arch Clin Exp Ophthalmol. 2022. PMID: 35384455 Review.
References
-
- Dohlman CH, Harissi-Dagher M, Khan BF, Sippel K, Aquavella JV, Graney JM. Introduction to the use of the Boston keratoprosthesis. Expert Review of Ophthalmology. 2006. October;1(1):41–8.
-
- Saeed HN, Shanbhag S, Chodosh J. The Boston keratoprosthesis. Curr Opin Ophthalmol. 2017. April 5; - PubMed
-
- Lee WB, Shtein RM, Kaufman SC, Deng SX, Rosenblatt MI. Boston Keratoprosthesis: Outcomes and Complications: A Report by the American Academy of Ophthalmology. Ophthalmology. 2015. July;122(7):1504–11. doi: 10.1016/j.ophtha.2015.03.025 - DOI - PubMed
-
- Zerbe BL, Belin MW, Ciolino JB, Boston Type 1 Keratoprosthesis Study Group. Results from the multicenter Boston Type 1 Keratoprosthesis Study. Ophthalmology. 2006. October;113(10):1779.e1–7. - PubMed
-
- Chew HF, Ayres BD, Hammersmith KM, Rapuano CJ, Laibson PR, Myers JS, et al. Boston keratoprosthesis outcomes and complications. Cornea. 2009. October;28(9):989–96. doi: 10.1097/ICO.0b013e3181a186dc - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

