Interpretation of correlated neural variability from models of feed-forward and recurrent circuits
- PMID: 29408930
- PMCID: PMC5833435
- DOI: 10.1371/journal.pcbi.1005979
Interpretation of correlated neural variability from models of feed-forward and recurrent circuits
Abstract
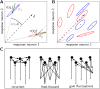
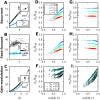
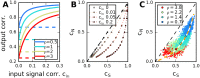
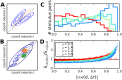
Neural populations respond to the repeated presentations of a sensory stimulus with correlated variability. These correlations have been studied in detail, with respect to their mechanistic origin, as well as their influence on stimulus discrimination and on the performance of population codes. A number of theoretical studies have endeavored to link network architecture to the nature of the correlations in neural activity. Here, we contribute to this effort: in models of circuits of stochastic neurons, we elucidate the implications of various network architectures-recurrent connections, shared feed-forward projections, and shared gain fluctuations-on the stimulus dependence in correlations. Specifically, we derive mathematical relations that specify the dependence of population-averaged covariances on firing rates, for different network architectures. In turn, these relations can be used to analyze data on population activity. We examine recordings from neural populations in mouse auditory cortex. We find that a recurrent network model with random effective connections captures the observed statistics. Furthermore, using our circuit model, we investigate the relation between network parameters, correlations, and how well different stimuli can be discriminated from one another based on the population activity. As such, our approach allows us to relate properties of the neural circuit to information processing.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
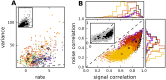


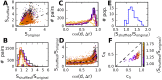
References
-
- Kriener B, Tetzlaff T, Aertsen A, Diesmann M, Rotter S. Correlations and Population Dynamics in Cortical Networks. Neural Comput. 2008;20(9):2185–2226. doi: 10.1162/neco.2008.02-07-474 - DOI - PubMed
-
- Ostojic S, Brunel N, Hakim V. How connectivity, background activity, and synaptic properties shape the cross-correlation between spike trains. J Neurosci. 2009;29(33):10234–53. doi: 10.1523/JNEUROSCI.1275-09.2009 - DOI - PMC - PubMed
-
- Tetzlaff T, Helias M, Einevoll GT, Diesmann M. Decorrelation of neural-network activity by inhibitory feedback. PLoS Comput Biol. 2012;8(8):e1002596 doi: 10.1371/journal.pcbi.1002596 - DOI - PMC - PubMed
-
- Trousdale J, Hu Y, Shea-Brown E, Josić K. Impact of network structure and cellular response on spike time correlations. PLoS Comput Biol. 2012;8(3):e1002408 doi: 10.1371/journal.pcbi.1002408 - DOI - PMC - PubMed
-
- Renart A, la Rocha JD, Bartho P. The asynchronous state in cortical circuits. Science. 2010;327(5965):587–590. doi: 10.1126/science.1179850 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

