Carbon nanofiber-filled conductive silicone elastomers as soft, dry bioelectronic interfaces
- PMID: 29408942
- PMCID: PMC5800568
- DOI: 10.1371/journal.pone.0189415
Carbon nanofiber-filled conductive silicone elastomers as soft, dry bioelectronic interfaces
Abstract
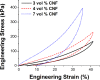
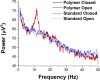
Soft and pliable conductive polymer composites hold promise for application as bioelectronic interfaces such as for electroencephalography (EEG). In clinical, laboratory, and real-world EEG there is a desire for dry, soft, and comfortable interfaces to the scalp that are capable of relaying the μV-level scalp potentials to signal processing electronics. A key challenge is that most material approaches are sensitive to deformation-induced shifts in electrical impedance associated with decreased signal-to-noise ratio. This is a particular concern in real-world environments where human motion is present. The entire set of brain information outside of tightly controlled laboratory or clinical settings are currently unobtainable due to this challenge. Here we explore the performance of an elastomeric material solution purposefully designed for dry, soft, comfortable scalp contact electrodes for EEG that is specifically targeted to have flat electrical impedance response to deformation to enable utilization in real world environments. A conductive carbon nanofiber filled polydimethylsiloxane (CNF-PDMS) elastomer was evaluated at three fill ratios (3, 4 and 7 volume percent). Electromechanical testing data is presented showing the influence of large compressive deformations on electrical impedance as well as the impact of filler loading on the elastomer stiffness. To evaluate usability for EEG, pre-recorded human EEG signals were replayed through the contact electrodes subjected to quasi-static compressive strains between zero and 35%. These tests show that conductive filler ratios well above the electrical percolation threshold are desirable in order to maximize signal-to-noise ratio and signal correlation with an ideal baseline. Increasing fill ratios yield increasingly flat electrical impedance response to large applied compressive deformations with a trade in increased material stiffness, and with nominal electrical impedance tunable over greater than 4 orders of magnitude. EEG performance was independent of filler loading above 4 vol % CNF (< 103 ohms).
Conflict of interest statement
Figures





Similar articles
-
Self-Adhesive and Capacitive Carbon Nanotube-Based Electrode to Record Electroencephalograph Signals From the Hairy Scalp.IEEE Trans Biomed Eng. 2016 Jan;63(1):138-47. doi: 10.1109/TBME.2015.2478406. Epub 2015 Sep 14. IEEE Trans Biomed Eng. 2016. PMID: 26390442
-
Carbonaceous Filler Type and Content Dependence of the Physical-Chemical and Electromechanical Properties of Thermoplastic Elastomer Polymer Composites.Materials (Basel). 2019 Apr 30;12(9):1405. doi: 10.3390/ma12091405. Materials (Basel). 2019. PMID: 31052175 Free PMC article.
-
Conductive polymer foam surface improves the performance of a capacitive EEG electrode.IEEE Trans Biomed Eng. 2012 Dec;59(12):3422-31. doi: 10.1109/TBME.2012.2215032. Epub 2012 Sep 3. IEEE Trans Biomed Eng. 2012. PMID: 22961261
-
Performance of conformable, dry EEG sensors.Annu Int Conf IEEE Eng Med Biol Soc. 2018 Jul;2018:4957-4960. doi: 10.1109/EMBC.2018.8513428. Annu Int Conf IEEE Eng Med Biol Soc. 2018. PMID: 30441455
-
The Current State of Silicone-Based Dielectric Elastomer Transducers.Macromol Rapid Commun. 2016 Mar;37(5):378-413. doi: 10.1002/marc.201500576. Epub 2016 Jan 15. Macromol Rapid Commun. 2016. PMID: 26773231 Review.
Cited by
-
A Review of Developments in Carbon-Based Nanocomposite Electrodes for Noninvasive Electroencephalography.Sensors (Basel). 2025 Apr 3;25(7):2274. doi: 10.3390/s25072274. Sensors (Basel). 2025. PMID: 40218785 Free PMC article. Review.
-
Flexible 3D-Printed EEG Electrodes.Sensors (Basel). 2019 Apr 6;19(7):1650. doi: 10.3390/s19071650. Sensors (Basel). 2019. PMID: 30959912 Free PMC article.
-
Emulation of the BIS engine.J Clin Monit Comput. 2022 Apr;36(2):483-492. doi: 10.1007/s10877-021-00676-2. Epub 2021 Mar 19. J Clin Monit Comput. 2022. PMID: 33742345 Free PMC article.
-
The Feature, Performance, and Prospect of Advanced Electrodes for Electroencephalogram.Biosensors (Basel). 2023 Jan 6;13(1):101. doi: 10.3390/bios13010101. Biosensors (Basel). 2023. PMID: 36671936 Free PMC article. Review.
-
Multi-Domain Convolutional Neural Networks for Lower-Limb Motor Imagery Using Dry vs. Wet Electrodes.Sensors (Basel). 2021 Oct 7;21(19):6672. doi: 10.3390/s21196672. Sensors (Basel). 2021. PMID: 34640992 Free PMC article.
References
-
- Ferree TC, Luu P, Russell GS, Tucker DM. Scalp electrode impedance, infection risk, and EEG data quality. Clin Neurophysiol. 2001;112: 536–544. doi: 10.1016/S1388-2457(00)00533-2 - DOI - PubMed
-
- McDowell K, Chin-Teng Lin, Oie KS, Tzyy-Ping Jung, Gordon S, Whitaker KW, et al. Real-World Neuroimaging Technologies. IEEE Access. 2013;1: 131–149. doi: 10.1109/ACCESS.2013.2260791 - DOI
-
- Lachaux J-P, Axmacher N, Mormann F, Halgren E, Crone NE. High-frequency neural activity and human cognition: Past, present and possible future of intracranial EEG research. Prog Neurobiol. 2012;98: 279–301. doi: 10.1016/j.pneurobio.2012.06.008 - DOI - PMC - PubMed
-
- Lal SKL, Craig A. Driver fatigue: Electroencephalography and psychological assessment. Psychophysiology. 2002;39: 313–321. doi: 10.1017.S0048577201393095 - PubMed
-
- Gevins A, Leong H, Du R, Smith ME, Le J, DuRousseau D, et al. Towards measurement of brain function in operational environments. Biol Psychol. 1995;40: 169–186. doi: 10.1016/0301-0511(95)05105-8 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

