Analysis of HBV basal core promoter/precore gene variability in patients with HBV drug resistance and HIV co-infection in Northwest Ethiopia
- PMID: 29408943
- PMCID: PMC5800642
- DOI: 10.1371/journal.pone.0191970
Analysis of HBV basal core promoter/precore gene variability in patients with HBV drug resistance and HIV co-infection in Northwest Ethiopia
Abstract
Background: We recently reported complex hepatitis B virus (HBV) drug resistant and concomitant vaccine escape hepatitis B surface antigen (HBsAg) variants during human immunodeficiency virus (HIV) co-infection and antiretroviral therapy (ART) exposure in Ethiopia. As a continuation of this report using the HBV positive sera from the same study participants, the current study further analyzed the HBV basal core promoter (BCP)/precore (PC) genes variability in patients with HBV drug resistance (at tyrosine-methionine-aspartate-aspartate (YMDD) reverse transcriptase (RT) motifs) and HIV co-infection in comparison with HBV mono-infected counterparts with no HBV drug resistant gene variants.
Materials and methods: A total of 143 participants of HBV-HIV co-infected (n = 48), HBV mono-infected blood donors (n = 43) and chronic liver disease (CLD) patients (n = 52) were included in the study. The BCP/PC genome regions responsible for HBeAg expression from the EcoRI site (nucleotides 1653-1959) were sequenced and analyzed for the BCP/PC mutant variants.
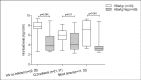
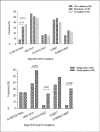
Results: Among the major mutant variants detected, double BCP mutations (A1762T/G1764A) (25.9%), Kozak sequences mutations (nt1809-1812) (51.7%) and the classical PC mutations such as A1814C/C1816T (15.4%), G1896A (25.2%) and G1862T (44.8%) were predominant mutant variants. The prevalence of the double BCP mutations was significantly lower in HIV co-infected patients (8.3%) compared with HBV mono-infected blood donors (32.6%) and CLD patients (36.5%). However, the Kozak sequences BCP mutations and the majority of PC mutations showed no significant differences among the study groups. Moreover, except for the overall BCP/PC mutant variants, co-prevalence rates of each major BCP/PC mutations and YMDDRT motif associated lamivudine (3TC)/entecavir (ETV) resistance mutations showed no significant differences when compared with the rates of BCP/PC mutations without YMDD RT motif drug resistance gene mutations. Unlike HIV co-infected group, no similar comparison made among HBV mono-infected blood donors and CLD patients since none of them developed the YMDD RT motif associated 3TC/ETV resistance mutations. However, HBV mono-infected blood donors and CLD patients who had no any drug resistance gene variants developed comparable G1862T (60.6% vs. 65.1%) and G1896A (24.2% vs. 11.6%) PC gene mutations.
Conclusion: No correlation observed between the BCP/PC genome variability and the YMDD RT motif associated HBV drug resistance gene variants during HIV co-infection. Nevertheless, irrespective of HIV co-infection status, the higher records of the BCP/PC gene variability in this study setting indicate a high risk of potential HBeAg negative chronic HBV infection in Northwest Ethiopia.
Conflict of interest statement
Figures
References
-
- Yuen MF, Sablon E, Yuan HJ, Hui CK, Wong DK, Doutreloigne J et al. (2002) Relationship between the development of precore and core promoter mutations and hepatitis B e antigen seroconversion in patients with chronic hepatitis B virus. J Infect Dis 186 (9): 1335–1338. doi: 10.1086/344327 - DOI - PubMed
-
- Chauhan R, Kazim SN, Bhattacharjee J, Sakhuja P, Sarin SK (2006) Basal core promoter, precore region mutations of HBV and their association with e antigen, genotype, and severity of liver disease in patients with chronic hepatitis B in India. J Med Virol 78 (8): 1047–1054. doi: 10.1002/jmv.20661 - DOI - PubMed
-
- Kamijo N, Matsumoto A, Umemura T, Shibata S, Ichikawa Y, Kimura T et al. (2015) Mutations of pre-core and basal core promoter before and after hepatitis B e antigen seroconversion. World J Gastroenterol 21 (2): 541–548. doi: 10.3748/wjg.v21.i2.541 - DOI - PMC - PubMed
-
- Alexopoulou A, Karayiannis P (2014) HBeAg negative variants and their role in the natural history of chronic hepatitis B virus infection. World J Gastroenterol 20 (24): 7644–7652. doi: 10.3748/wjg.v20.i24.7644 - DOI - PMC - PubMed
-
- Ghosh S, Mondal RK, Banerjee P, Nandi M, Sarkar S, Das K et al. (2012) Tracking the naturally occurring mutations across the full-length genome of hepatitis B virus of genotype D in different phases of chronic e-antigen-negative infection. Clin Microbiol Infect 18 (10): E412–8. doi: 10.1111/j.1469-0691.2012.03975.x - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

