Better living through chemistry: Addressing emerging antibiotic resistance
- PMID: 29409348
- PMCID: PMC5882019
- DOI: 10.1177/1535370218755659
Better living through chemistry: Addressing emerging antibiotic resistance
Abstract
The increasing emergence of multidrug-resistant bacteria is recognized as a major threat to human health worldwide. While the use of small molecule antibiotics has enabled many modern medical advances, it has also facilitated the development of resistant organisms. This minireview provides an overview of current small molecule drugs approved by the US Food and Drug Administration (FDA) for use in humans, the unintended consequences of antibiotic use, and the mechanisms that underlie the development of drug resistance. Promising new approaches and strategies to counter antibiotic-resistant bacteria with small molecules are highlighted. However, continued public investment in this area is critical to maintain an edge in our evolutionary "arms race" against antibiotic-resistant microorganisms. Impact statement The alarming increase in antibiotic-resistant microorganisms is a rapidly emerging threat to human health throughout the world. Historically, small molecule drugs have played a major role in controlling bacterial infections and they continue to offer tremendous potential in countering resistant organisms. This minireview provides a broad overview of the relevant issues, including the diversity of FDA-approved small molecule drugs and mechanisms of drug resistance, unintended consequences of antibiotic use, the current state of development for small molecule antibacterials and financial challenges that impact progress towards novel therapies. The content will be informative to diverse stakeholders, including clinicians, basic scientists, translational scientists and policy makers, and may be used as a bridge between these key players to advance the development of much-needed therapeutics.
Keywords: Bacteria; drugs; mechanisms; pharmacology; resistance; small molecule inhibitors.
Figures
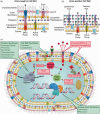
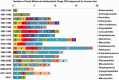

References
-
- FDIS I. 11238 Health informatics – identification of medicinal products – data elements and structures for the unique identification and exchange of regulated information on substances. Available at: http://bit ly/obxMhU (accessed October 2011)
-
- Infectious Diseases Society of A, Spellberg B, Blaser M, Guidos RJ, Boucher HW, Bradley JS, Eisenstein BI, Gerding D, Lynfield R, Reller LB, Rex J, Schwartz D, Septimus E, Tenover FC, Gilbert DN. Combating antimicrobial resistance: policy recommendations to save lives. Clin Infect Dis 2011; 52:S397–428 - PMC - PubMed
-
- Eswari JS, Dhagat S, Kaser S, Tiwari A. Homology modeling and molecular docking studies of Bacillomycin and Iturin synthetases with novel ligands for the production of therapeutic lipopeptides. Curr Drug Discov Technol. Epub ahead of print 15 August 2017. doi: 10.2174/1570163814666170816112536. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

