Using an onset-anchored Bayesian hierarchical model to improve predictions for amyotrophic lateral sclerosis disease progression
- PMID: 29409450
- PMCID: PMC5801819
- DOI: 10.1186/s12874-018-0479-9
Using an onset-anchored Bayesian hierarchical model to improve predictions for amyotrophic lateral sclerosis disease progression
Abstract
Background: Amyotrophic Lateral Sclerosis (ALS), also known as Lou Gehrig's disease, is a rare disease with extreme between-subject variability, especially with respect to rate of disease progression. This makes modelling a subject's disease progression, which is measured by the ALS Functional Rating Scale (ALSFRS), very difficult. Consider the problem of predicting a subject's ALSFRS score at 9 or 12 months after a given time-point.
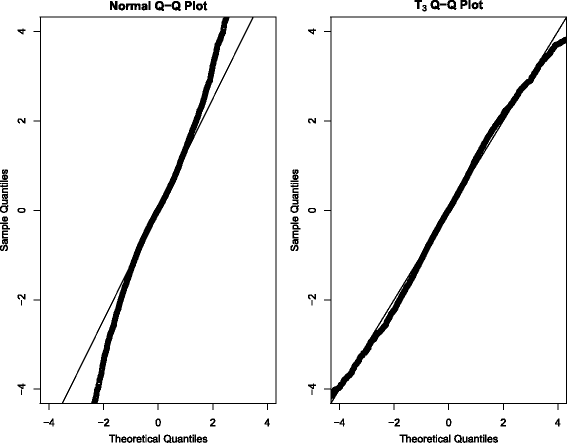
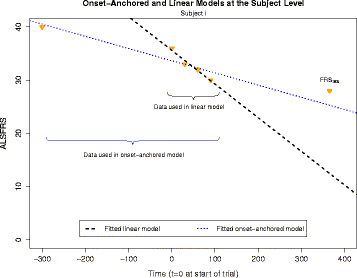
Methods: We obtained ALS subject data from the Pooled Resource Open-Access ALS Clinical Trials Database, a collection of data from various ALS clinical trials. Due to the typical linearity of the ALSFRS, we consider several Bayesian hierarchical linear models. These include a mixture model (to account for the two potential classes of "fast" and "slow" ALS progressors) as well as an onset-anchored model, in which an additional artificial data-point, using time of disease onset, is utilized to improve predictive performance.
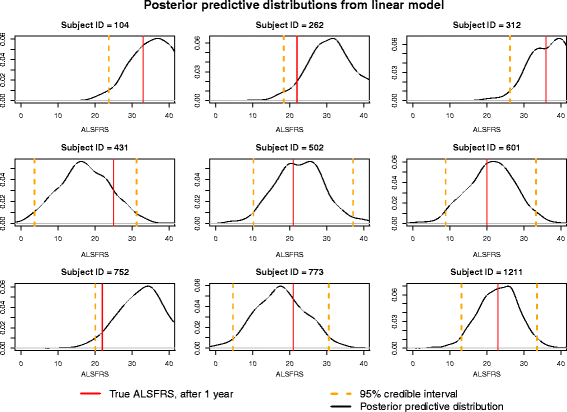
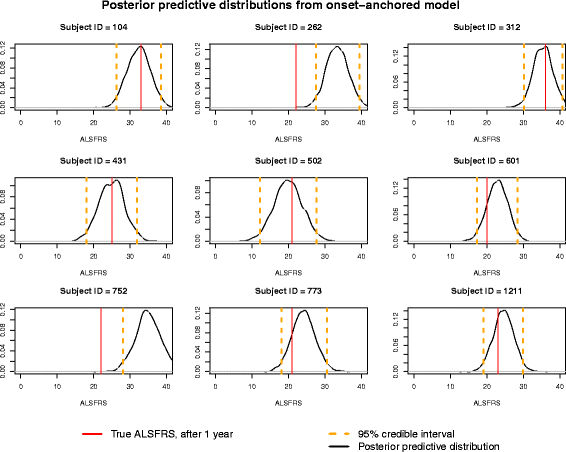
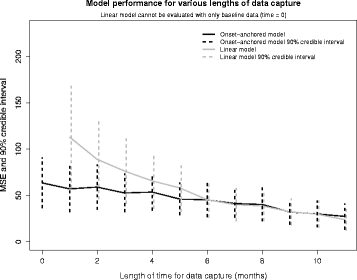
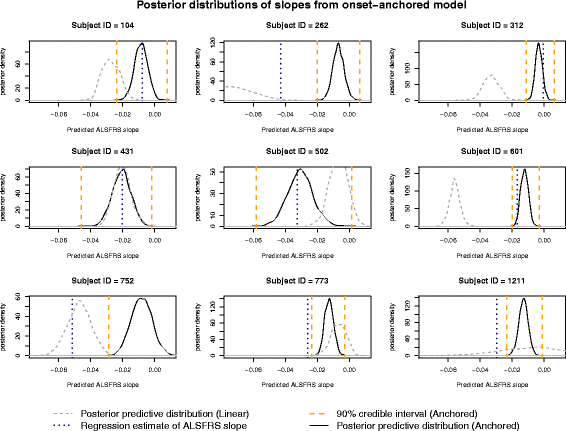
Results: The onset-anchored model had a drastically reduced posterior predictive mean-square-error distributions, when compared to the Bayesian hierarchical linear model or the mixture model under a cross-validation approach. No covariates, other than time of disease onset, consistently improved predictive performance in either the Bayesian hierarchical linear model or the onset-anchored model.
Conclusions: Augmenting patient data with an additional artificial data-point, or onset anchor, can drastically improve predictive modelling in ALS by reducing the variability of estimated parameters at the cost of a slight increase in bias. This onset-anchored model is extremely useful if predictions are desired directly after a single baseline measure (such as at the first day of a clinical trial), a feat that would be very difficult without the onset-anchor. This approach could be useful in modelling other diseases that have bounded progression scales (e.g. Parkinson's disease, Huntington's disease, or inclusion-body myositis). It is our hope that this model can be used by clinicians and statisticians to improve the efficacy of clinical trials and aid in finding treatments for ALS.
Keywords: ALS; ALSFRS; Hierarchical modelling; Onset-anchor; Prediction; Pro-act.
Conflict of interest statement
Ethics approval and consent to participate
In all of the trials that generated the data included in this database, study protocols were approved by the participating medical centers and all participating patients gave informed consent. De-identified data from these trials were donated to the PRO-ACT database for research purposes only and under the explicit conditions that Prize4Life and all users of the data would maintain the anonymity of subjects and not attempt to discover the identity of any subject. In the rare cases where donated data was not already completely anonymized, donated data was further anonymized following the HIPAA de-identification conventions for personal health information: any potential patient initials and/or dates of birth were removed, new randomized subject numbers were created, and wherever possible, trial-specific information was removed in the merging of datasets, including trial center identity and location, trial dates, or other identifying information. This project was deemed not to constitute human subjects research and the need for ethical approval for this work was waived by the University of Kansas Medical Center’s Human Research Protection Program.
Consent for publication
Not applicable
Competing interests
Jeffrey M. Statland is a consultant for aTyr, Acceleron, Fulcrum, Regeneron, and Strongbridge. The remaining authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Ikeda K, Hirayama T, Takazawa T, Kawabe K, Iwasaki Y. Relationships between disease progression and serum levels of lipid, urate, creatinine and ferritin in Japanese patients with amyotrophic lateral sclerosis: a cross-sectional study. Intern Med. 2012;51(12):1501–1508. doi: 10.2169/internalmedicine.51.7465. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

