Fate of Antibody-Drug Conjugates in Cancer Cells
- PMID: 29409507
- PMCID: PMC5802061
- DOI: 10.1186/s13046-017-0667-1
Fate of Antibody-Drug Conjugates in Cancer Cells
Abstract
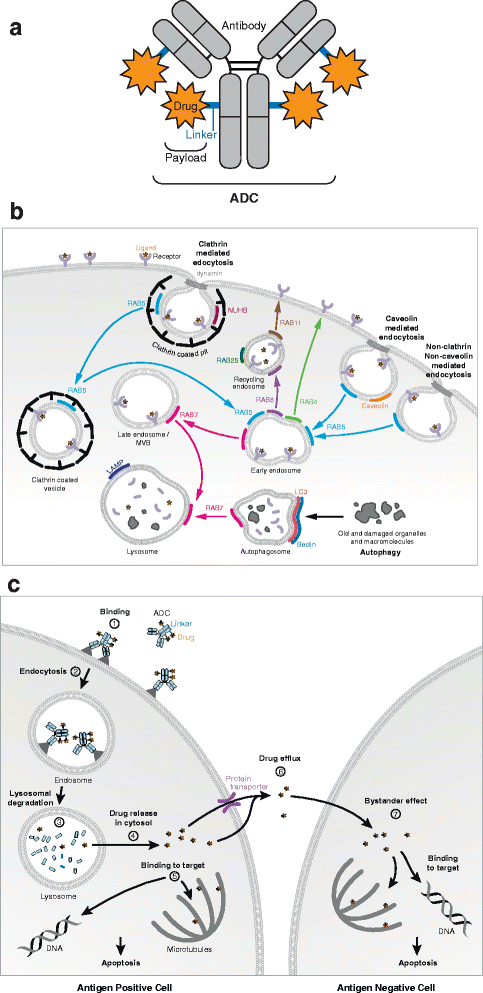
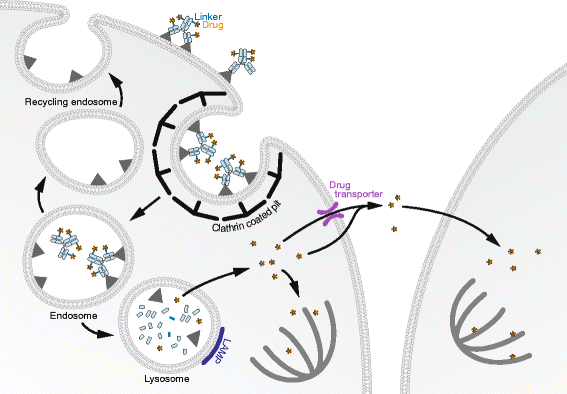
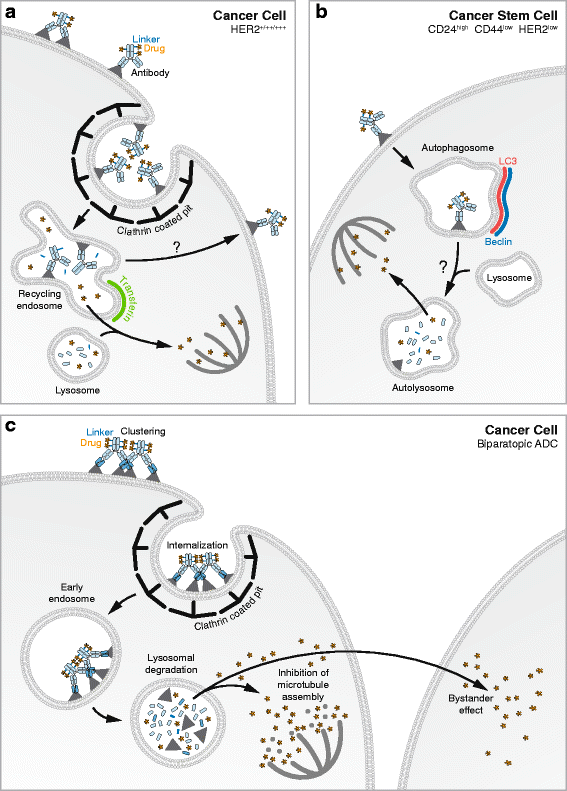
Antibody-Drug Conjugates (ADCs) are a class of cancer therapeutics that combines antigen specificity and potent cytotoxicity in a single molecule as they are comprised of an engineered antibody linked chemically to a cytotoxic drug. Four ADCs have received approval by the Food and Drug Administration (FDA) and the European Medicine Agency (EMA) and can be prescribed for metastatic conditions while around 60 ADCs are currently enrolled in clinical trials. The efficacy of an ADC greatly relies on its intracellular trafficking and processing of its components to trigger tumor cell death. A limited number of studies have addressed these critical processes that both challenge and help foster the design of ADCs. This review highlights those mechanisms and their relevance for future development of ADCs as cancer therapeutics.
Keywords: Antibody-drug conjugates; Endocytic compartments; Endocytosis; Intracellular trafficking.
Conflict of interest statement
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

