Mesenchymal stromal cell-derived exosome-rich fractionated secretome confers a hepatoprotective effect in liver injury
- PMID: 29409540
- PMCID: PMC5801895
- DOI: 10.1186/s13287-017-0752-6
Mesenchymal stromal cell-derived exosome-rich fractionated secretome confers a hepatoprotective effect in liver injury
Abstract
Background: Mesenchymal stromal cells (MSCs) are an attractive therapeutic agent in regenerative medicine. Recently, there has been a paradigm shift from differentiation of MSCs to their paracrine effects at the injury site. Several reports elucidate the role of trophic factors secreted by MSCs toward the repair of injured tissues. We hypothesize that fractionating the MSC secretome will enrich exosomes containing soluble bioactive molecules, improving its therapeutic potential for liver failure.
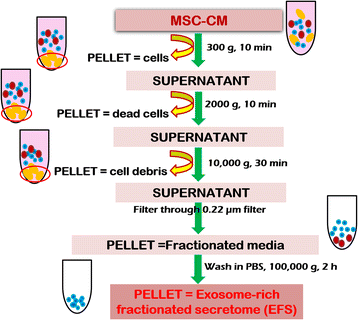
Methods: Rat bone marrow MSCs were isolated and the conditioned media filtered, concentrated and ultracentrifuged to generate fractionated secretome. This secretome was characterized for the presence of exosomes and recovery from liver injury assessed in in-vitro liver injury models. The results were further validated in vivo.
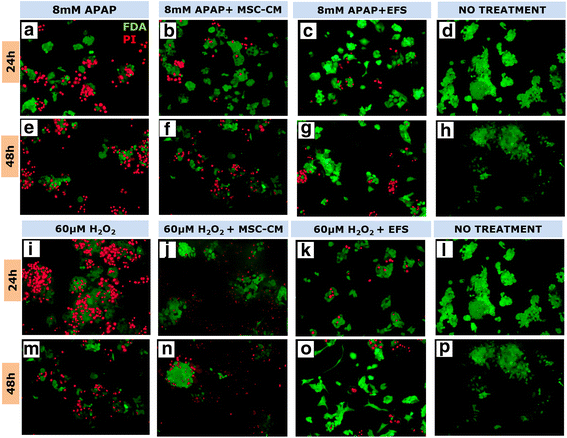
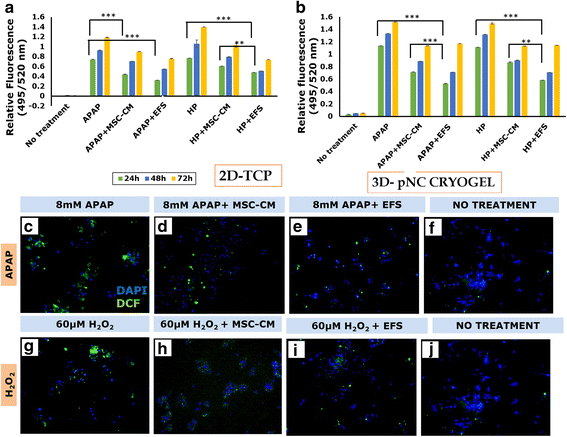
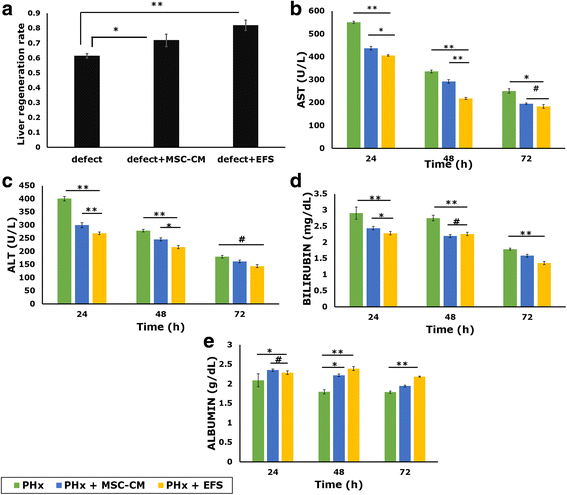
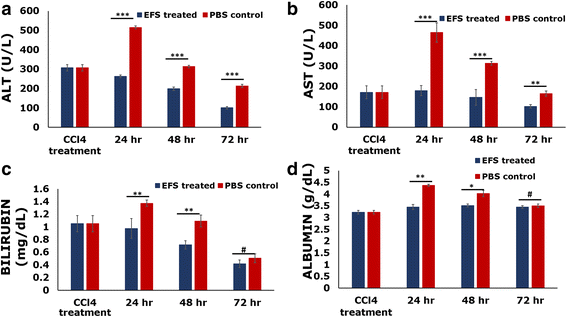
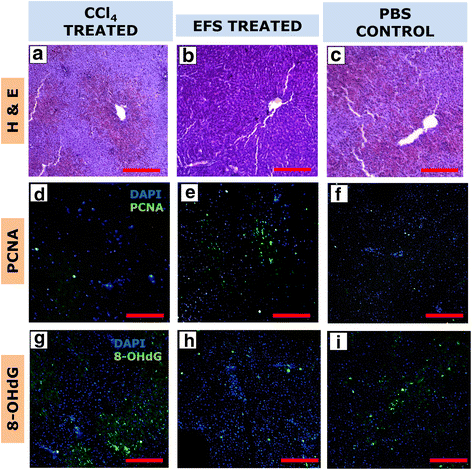
Results: Studies on in-vitro liver injury models using acetaminophen and hydrogen peroxide show better cell recovery and reduced cytotoxicity in the presence of fractionated as opposed to unfractionated secretome. Further, the cells showed reduced oxidative stress in the presence of fractionated secretome, suggesting a potential antioxidative effect. These results were further validated in vivo in liver failure models, wherein improved liver regeneration in the presence of fractionated secretome (0.819 ± 0.035) was observed as compared to unfractionated secretome (0.718 ± 0.042).
Conclusions: The work presented is a proof of concept that fractionating the secretome enriches certain bioactive molecules involved in the repair and recovery of injured liver tissue. Exosome enriched mesenchymal stromal cell-derived fractionated secretome potentiates recovery upon injection in injured liver.
Keywords: Cryogel; Exosomes; Liver; Secretome; Stromal cells.
Conflict of interest statement
Ethics approval and consent to participate
Isolation of bone marrow-derived MSCs and development of ALF models were carried out using protocols approved by the Institute Animal Ethics Committee (IITK/IAEC/2014/1023 and IITK/IAEC/2014/1022, respectively) of IIT Kanpur, under the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India. All methods were performed in accordance with relevant guidelines and regulations of this committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Canbay A, Tacke F, Hadem J, Trautwein C, Gerken G, Manns MP. Acute Liver Failure: A Life-Threatening Disease. Dtsch Arztebl Int. 2011;108(42):714–20. http://doi.org/10.3238/arztebl.2011.0714. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

