The Effect of Selective c-MET Inhibitor on Hepatocellular Carcinoma in the MET-Active, β-Catenin-Mutated Mouse Model
- PMID: 29409568
- PMCID: PMC5954626
- DOI: 10.3727/105221618X15174108894682
The Effect of Selective c-MET Inhibitor on Hepatocellular Carcinoma in the MET-Active, β-Catenin-Mutated Mouse Model
Abstract
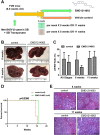
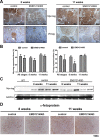
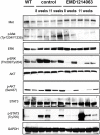
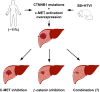
Simultaneous mutations in CTNNB1 and activation of c-MET occur in 9%-12.5% of patients with hepatocellular carcinoma (HCC). Coexpression of c-MET-V5 and mutant β-catenin-Myc in mouse liver by sleeping beauty transposon/transposase and hydrodynamic tail vein injection (SB-HTVI) led to the development of HCC with 70% molecular identity to the clinical subset. Using this model, we investigated the effect of EMD1214063, a highly selective c-MET inhibitor. Five weeks after SB-HTVI when tumors were established, EMD1214063 (10 mg/kg) was administered by gastric gavage as a single agent on 5-day-on/3-day-off schedule, compared to vehicle only control. Mice were harvested at 8 or 11 weeks posttreatment. Decreased p-MET, p-AKT, p-STAT3, and p-ERK proved in vivo efficacy of EMD1214063. We observed lower Ki-67, PCNA, V5-tag, and cyclin D1 after EMD1214063 treatment only at 8 weeks. Overall, no significant differences were observed in tumor burden between the groups, although EMD1214063 marginally but significantly improved overall survival by 1.5-2 weeks. Tumors remained α-fetoprotein+, did not show any differences in inflammation, and lacked fibrosis in either group. In conclusion, c-MET inhibition alone had a minor effect on Met-β-catenin HCC at the early stages of HCC development. Thus, a single therapy with the c-MET inhibitor will be insufficient for sustained response in Met-β-catenin HCC requiring assessment of additional combinations.
Conflict of interest statement
The authors declare no conflicts of interests.
Figures



References
-
- El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118–27. - PubMed
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. - PubMed
-
- 2017 Cancer stat facts: Liver and intrahepatic bile duct cancer. Available at https://seer.cancer.gov/statfacts/html/livibd.html
-
- Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G; RESORCE Investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389(10064):56–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
