Early season co-circulation of influenza A(H3N2) and B(Yamagata): interim estimates of 2017/18 vaccine effectiveness, Canada, January 2018
- PMID: 29409570
- PMCID: PMC5801641
- DOI: 10.2807/1560-7917.ES.2018.23.5.18-00035
Early season co-circulation of influenza A(H3N2) and B(Yamagata): interim estimates of 2017/18 vaccine effectiveness, Canada, January 2018
Erratum in
-
Authors' correction for Euro Surveill. 2018;23(5).Euro Surveill. 2018 Jul;23(30):1807261. doi: 10.2807/1560-7917.ES.2018.23.30.1807261. Euro Surveill. 2018. PMID: 30064546 Free PMC article. No abstract available.
Abstract
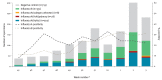
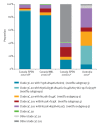
Using a test-negative design, we assessed interim vaccine effectiveness (VE) for the 2017/18 epidemic of co-circulating influenza A(H3N2) and B(Yamagata) viruses. Adjusted VE for influenza A(H3N2), driven by a predominant subgroup of clade 3C.2a viruses with T131K + R142K + R261Q substitutions, was low at 17% (95% confidence interval (CI): -14 to 40). Adjusted VE for influenza B was higher at 55% (95% CI: 38 to 68) despite prominent use of trivalent vaccine containing lineage-mismatched influenza B(Victoria) antigen, suggesting cross-lineage protection.
Keywords: Influenza; genomics; influenza virus; mid-season; vaccine effectiveness; vaccine-preventable diseases; vaccines and immunisation.
Conflict of interest statement
Figures
References
-
- Public Health Agency of Canada (PHAC). FluWatch: Influenza weekly reports 2017-18 season. Ottawa: PHAC; 2018. [Accessed 15 January 2018]. Available from: https://www.canada.ca/en/public-health/services/diseases/flu-influenza/i....
-
- European Centre for Disease Prevention and Control (ECDC). Flu New Europe: Joint ECDC-WHO/Europe weekly influenza update. [Accessed 15 January 2018]. Stockholm: ECDC; 2018. Available from: http://flunewseurope.org/.
-
- Centers for Disease Control and Prevention (CDC). FluView: Weekly U.S. influenza surveillance report. Atlanta: CDC; 2018. [Accessed 15 January 2018]. Available from: https://www.cdc.gov/flu/weekly/.
-
- World Health Organization (WHO). WHO recommendations on the composition of influenza virus vaccines. Geneva: WHO. Available from: http://www.who.int/influenza/vaccines/virus/recommendations/en/
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
