In vivo Raman spectroscopy for biochemical monitoring of the human cervix throughout pregnancy
- PMID: 29410109
- PMCID: PMC5916496
- DOI: 10.1016/j.ajog.2018.01.030
In vivo Raman spectroscopy for biochemical monitoring of the human cervix throughout pregnancy
Abstract
Background: The cervix must undergo significant biochemical remodeling to allow for successful parturition. This process is not fully understood, especially in instances of spontaneous preterm birth. In vivo Raman spectroscopy is an optical technique that can be used to investigate the biochemical composition of tissue longitudinally and noninvasively in human beings, and has been utilized to measure physiology and disease states in a variety of medical applications.
Objective: The purpose of this study is to measure in vivo Raman spectra of the cervix throughout pregnancy in women, and to identify biochemical markers that change with the preparation for delivery and postpartum repair.
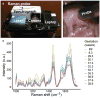
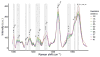
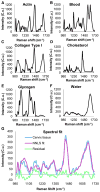
Study design: In all, 68 healthy pregnant women were recruited. Raman spectra were measured from the cervix of each patient monthly in the first and second trimesters, weekly in the third trimester, and at the 6-week postpartum visit. Raman spectra were measured using an in vivo Raman system with an optical fiber probe to excite the tissue with 785 nm light. A spectral model was developed to highlight spectral regions that undergo the most changes throughout pregnancy, which were subsequently used for identifying Raman peaks for further analysis. These peaks were analyzed longitudinally to determine if they underwent significant changes over the course of pregnancy (P < .05). Finally, 6 individual components that comprise key biochemical constituents of the human cervix were measured to extract their contributions in spectral changes throughout pregnancy using a linear combination method. Patient factors including body mass index and parity were included as variables in these analyses.
Results: Raman peaks indicative of extracellular matrix proteins (1248 and 1254 cm-1) significantly decreased (P < .05), while peaks corresponding to blood (1233 and 1563 cm-1) significantly increased (P < .0005) in a linear manner throughout pregnancy. In the postpartum cervix, significant increases in peaks corresponding to actin (1003, 1339, and 1657 cm-1) and cholesterol (1447 cm-1) were observed when compared to late gestation, while signatures from blood significantly decreased. Postpartum actin signals were significantly higher than early pregnancy, whereas extracellular matrix proteins and water signals were significantly lower than early weeks of gestation. Parity had a significant effect on blood and extracellular matrix protein signals, with nulliparous patients having significant increases in blood signals throughout pregnancy, and higher extracellular matrix protein signals in early pregnancy compared to patients with prior pregnancies. Body mass index significantly affected actin signal contribution, with low body mass index patients showing decreasing actin contribution throughout pregnancy and high body mass index patients demonstrating increasing actin signals.
Conclusion: Raman spectroscopy was successfully used to biochemically monitor cervical remodeling in pregnant women during prenatal visits. This foundational study has demonstrated sensitivity to known biochemical dynamics that occur during cervical remodeling, and identified patient variables that have significant effects on Raman spectra throughout pregnancy. Raman spectroscopy has the potential to improve our understanding of cervical maturation, and be used as a noninvasive preterm birth risk assessment tool to reduce the incidence, morbidity, and mortality caused by preterm birth.
Keywords: Raman spectroscopy; cervical remodeling; in vivo; optical spectroscopy; postpartum; pregnancy.
Copyright © 2018. Published by Elsevier Inc.
Conflict of interest statement
The authors report no conflict of interest.
Figures
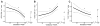

References
-
- Macdonald PC, Gant NF, Leveno KJ, Gilstrap LC, Hankins G, Clark SL. Williams obstetrics. 20. Stamford, CT: Appleton and Lange; 1996.
-
- Ludmir J, Sehdev HM. Anatomy and physiology of the uterine cervix. Clin Obstet Gynecol. 2000;43:433–9. - PubMed
-
- Vink JY, Qin S, Brock CO, et al. A new paradigm for the role of smooth muscle cells in the human cervix. Am J Obstet Gynecol. 2016;215:478e1–11. - PubMed
-
- Word RA, Li XH, Hnat M, Carrick K. Dynamics of cervical remodeling during pregnancy and parturition: mechanisms and current concepts. Semin Reprod Med. 2007;25:69–79. - PubMed
-
- Friedman EA, Kroll BH. Computer analysis of labor progression. 3. Pattern variations by parity. J Reprod Med. 1971;6:179–83. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

