Plerixafor Plus Granulocyte Colony-Stimulating Factor for Patients with Non-Hodgkin Lymphoma and Multiple Myeloma: Long-Term Follow-Up Report
- PMID: 29410180
- PMCID: PMC6091693
- DOI: 10.1016/j.bbmt.2018.01.039
Plerixafor Plus Granulocyte Colony-Stimulating Factor for Patients with Non-Hodgkin Lymphoma and Multiple Myeloma: Long-Term Follow-Up Report
Abstract
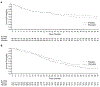
The purpose of this report is to analyze long-term clinical outcomes of patients exposed to plerixafor plus granulocyte colony-stimulating factor (G-CSF) for stem cell mobilization. This was a study of patients with non-Hodgkin lymphoma (NHL; n = 167) and multiple myeloma (MM; n = 163) who were enrolled in the long-term follow-up of 2 pivotal phase III studies (NCT00741325 and NCT00741780) of 240 µg/kg plerixafor plus 10 µg/kg G-CSF, or placebo plus 10 µg/kg G-CSF to mobilize and collect CD34+ cells for autologous hematopoietic stem cell transplantation. Overall survival (OS) and progression-free survival (PFS) were evaluated over a 5-year period following the first dose of plerixafor or placebo. The probability of OS was not significantly different in patients with NHL or MM treated with plerixafor or placebo (NHL: 64%; 95% confidence interval [CI], 56% to 71% versus 56%; 95% CI, 44% to 67%, respectively; MM: 64%; 95% CI, 54% to 72% versus 64%; 95% CI, 53% to 73%, respectively). In addition, there was no statistically significant difference in the probability of PFS over 5 years between treatment groups in patients with NHL (50%; 95% CI, 44% to 67% for plerixafor versus 43%; 95% CI, 31% to 54% for placebo) or those with MM (17%; 95% CI, 10% to 24% for plerixafor versus 30%; 95% CI, 21% to 40% for placebo). In this long-term follow-up study, the addition of plerixafor to G-CSF for stem cell mobilization did not affect 5-year survival in patients with NHL or patients with MM.
Keywords: Long-term follow-up; Multiple myeloma; Non-Hodgkin lymphoma; Plerixafor; Stem cell mobilization.
Copyright © 2018. Published by Elsevier Inc.
Figures



References
-
- Bensinger W, DiPersio JF, McCarty JM. Improving stem cell mobilization strategies: future directions. Bone Marrow Transplant. 2009;43:181–195. - PubMed
-
- Flomenberg N, DiPersio J, Calandra G. Role of CXCR4 chemokine receptor blockade using AMD3100 for mobilization of autologous hematopoietic progenitor cells. Acta Haematol. 2005;114:198–205. - PubMed
-
- Josefsen D, Rechnitzer C, Parto K, Kvalheim G. The use of Plerixafor for peripheral blood stem cell mobilisation reduces the frequency of mobilisation failure in patients planned to undergo autologous transplantation. European Haematology. 2010;4:24–29.
-
- Sehn LH, Fenske TS, Laport GG. Follicular lymphoma: prognostic factors, conventional therapies, and hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:S82–91. - PubMed
-
- Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med 2003;348:1875–1883. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

