Intestinal microbiota development and gestational age in preterm neonates
- PMID: 29410448
- PMCID: PMC5802739
- DOI: 10.1038/s41598-018-20827-x
Intestinal microbiota development and gestational age in preterm neonates
Abstract
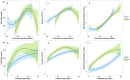
The intestinal microbiota is an important contributor to the health of preterm infants, and may be destabilized by a number of environmental factors and treatment modalities. How to promote the development of a healthy microbiota in preterm infants is largely unknown. We collected fecal samples from 45 breastfed preterm very low birth weight (birth weight < 1500 g) infants from birth until 60 days postnatal age to characterize the intestinal microbiota development during the first weeks of life in preterm infants. Fecal microbiota composition was determined by 16S rRNA amplicon sequencing. The main driver of microbiota development was gestational age; antibiotic use had strong but temporary effects and birth mode had little influence. Microbiota development proceeded in four phases indicated by the dominance of Staphylococcus, Enterococcus, Enterobacter, and finally Bifidobacterium. The Enterococcus phase was only observed among the extremely premature infants and appeared to delay the microbiota succession. The results indicate that hospitalized preterm infants receiving breast milk may develop a normal microbiota resembling that of term infants.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Neu, J., Chen, M. & Beierle, E. Intestinal innate immunity: how does it relate to the pathogenesis of necrotizing enterocolitis. In: Seminars in pediatric surgerysurgery, pp.137–144: Elsevier (2005). - PubMed
-
- Magne F, et al. Low species diversity and high interindividual variability in faeces of preterm infants as revealed by sequences of 16S rRNA genes and PCR-temporal temperature gradient gel electrophoresis profiles. FEMS Microbiol Ecol. 2006;57:128–138. doi: 10.1111/j.1574-6941.2006.00097.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

