Identification of a novel Na+-coupled Fe3+-citrate transport system, distinct from mammalian INDY, for uptake of citrate in mammalian cells
- PMID: 29410496
- PMCID: PMC5802838
- DOI: 10.1038/s41598-018-20620-w
Identification of a novel Na+-coupled Fe3+-citrate transport system, distinct from mammalian INDY, for uptake of citrate in mammalian cells
Retraction in
-
Retraction Note: Identification of a novel Na+-coupled Fe3+-citrate transport system, distinct from mammalian INDY, for uptake of citrate in mammalian cells.Sci Rep. 2018 Jul 17;8(1):11021. doi: 10.1038/s41598-018-28645-x. Sci Rep. 2018. PMID: 30018353 Free PMC article.
Abstract
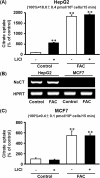
NaCT is a Na+-coupled transporter for citrate expressed in hepatocytes and neurons. It is the mammalian ortholog of INDY (I'm Not Dead Yet), a transporter which modifies lifespan in Drosophila. Here we describe a hitherto unknown transport system for citrate in mammalian cells. When liver and mammary epithelial cells were pretreated with the iron supplement ferric ammonium citrate (FAC), uptake of citrate increased >10-fold. Iron chelators abrogated the stimulation of citrate uptake in FAC-treated cells. The iron exporter ferroportin had no role in this process. The stimulation of citrate uptake also occurred when Fe3+ was added during uptake without pretreatment. Similarly, uptake of Fe3+ was enhanced by citrate. The Fe3+-citrate uptake was coupled to Na+. This transport system was detectable in primary hepatocytes and neuronal cell lines. The functional features of this citrate transport system distinguish it from NaCT. Loss-of-function mutations in NaCT cause early-onset epilepsy and encephalopathy; the newly discovered Na+-coupled Fe3+-citrate transport system might offer a novel treatment strategy for these patients to deliver citrate into affected neurons independent of NaCT. It also has implications to iron-overload conditions where circulating free iron increases, which would stimulate cellular uptake of citrate and consequently affect multiple metabolic pathways.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures








References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

