Salmonella Immunotherapy Improves the Outcome of CHOP Chemotherapy in Non-Hodgkin Lymphoma-Bearing Mice
- PMID: 29410666
- PMCID: PMC5787062
- DOI: 10.3389/fimmu.2018.00007
Salmonella Immunotherapy Improves the Outcome of CHOP Chemotherapy in Non-Hodgkin Lymphoma-Bearing Mice
Abstract
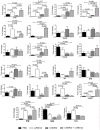
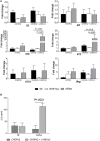
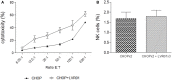
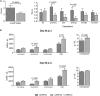
We have previously shown that Salmonella immunotherapy is effective to treat B-cell non-Hodgkin lymphoma (B-NHL) in mice. However, this model involves animals with high tumor burden, whereas in the clinics B-NHL patients are usually treated with chemotherapy (CHOP: cyclophosphamide, doxorubicin, vincristine, and prednisone) as first-line therapy prior to immunotherapy. Recently, we have described a NHL-B preclinical model using CHOP chemotherapy to achieve MRD in immunocompetent animals that closely resemble patients' conditions. In this work, we assessed the efficacy of Salmonella immunotherapy in B-NHL-bearing mice undergoing chemotherapy. Salmonella administration significantly delayed tumor growth and prolonged survival of chemotherapy-treated NHL-bearing animals. Mice receiving the CHOP-Salmonella combined therapy showed increased numbers of tumor-infiltrating leukocytes and a different profile of cytokines and chemokines expressed in the tumor microenvironment. Further, Salmonella immunotherapy in CHOP-treated animals also enhanced NK cells cytotoxic activity as well as induced systemic lymphoma-specific humoral and cellular responses. Chemotherapy treatment profoundly impacted on the general health status of recipient animals, but those receiving Salmonella showed significantly better overall body condition. Altogether, the results clearly demonstrated that Salmonella immunotherapy could be safely used in individuals under CHOP treatment, resulting in a better prognosis. These results give strong support to consider Salmonella as a neoadjuvant therapy in a clinical setting.
Keywords: CHOP; Immunotherapy; Salmonella; chemotherapy; non-Hodgkin lymphoma.
Figures




References
-
- Zelenetz AD, Gordon LI, Wierda WG, Abramson JS, Advani RH, Andreadis CB, et al. Non-Hodgkin’s lymphomas. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines), Version 4. National Comprehensive Cancer Network; (2014). Available from: https://www.nccn.org/about/nhl.pdf - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

