A Randomized Placebo Controlled Clinical Trial to Determine the Impact of Digestion Resistant Starch MSPrebiotic® on Glucose, Insulin, and Insulin Resistance in Elderly and Mid-Age Adults
- PMID: 29410955
- PMCID: PMC5787146
- DOI: 10.3389/fmed.2017.00260
A Randomized Placebo Controlled Clinical Trial to Determine the Impact of Digestion Resistant Starch MSPrebiotic® on Glucose, Insulin, and Insulin Resistance in Elderly and Mid-Age Adults
Abstract
Introduction: Type 2 diabetes (T2D) has reached epidemic proportions in North America. Recent evidence suggests that prebiotics can modulate the gut microbiome, which then plays an important role in regulating lipid metabolism, blood glucose, and insulin sensitivity. As such, prebiotics are appealing potential therapeutic strategies for prediabetes and T2D. The key objectives of this study were to determine the tolerability as well as the glucose and insulin modulating ability of MSPrebiotic® digestion resistant starch (DRS) in healthy mid-age (MID) and elderly (ELD) adults.
Materials and methods: This was a prospective, blinded, placebo-controlled study. Prediabetes and diabetes were among the exclusion factors. ELD (>70 years) and MID (30-50 years) Canadian adults were recruited and, after 2 weeks of consuming placebo, they were randomized to consume 30 g of either MSPrebiotic® or placebo per day for 12 weeks. In total, 42 ELD and 42 MID participants completed the study. Blood samples were collected over the 14-week study and analyzed for glucose, lipid profile, and CRP, lipid particles, TNF-α, IL-10, insulin, and insulin resistance (IR).
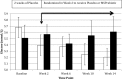
Results: At baseline, the ELD population had a significantly higher percentage (p < 0.01) with elevated glucose and significantly higher TNF-α (p < 0.01) compared to MID adults. MSPrebiotic® DRS was well tolerated in both MID and ELD adults. There was a significant difference over time in blood glucose (p = 0.0301) and insulin levels (p = 0.009), as well as IR (HOMA-IR; p = 0.009) in ELD adults who consumed MSPrebiotic® compared to placebo. No significant changes were found in MID adults.
Conclusion: Our results suggest that dietary supplementation with prebiotics such as MSPrebiotic® may be part of an effective strategy to reduce IR, a major risk factor for developing T2D, in the ELD.
Clinical trial registration: NCT01977183 listed on NIH website: ClinicalTrials.gov, The metadata generated in this study have been submitted to the NCBI Sequence Read Archive (http://www.ncbi.nlm.nih.gov/bioproject/381931).
Keywords: diabetes; digestion resistant starch; elderly; glucose; gut microbiome; insulin-resistance; prebiotics.
Figures


References
-
- Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol (2017). 14(8):491–502.10.1038/nrgastro.2017.75 - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

