The potential of antisense oligonucleotide therapies for inherited childhood lung diseases
- PMID: 29411170
- PMCID: PMC5801198
- DOI: 10.1186/s40348-018-0081-6
The potential of antisense oligonucleotide therapies for inherited childhood lung diseases
Abstract
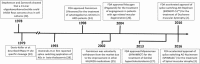
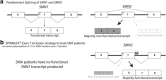
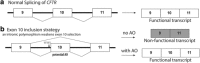
Antisense oligonucleotides are an emerging therapeutic option to treat diseases with known genetic origin. In the age of personalised medicines, antisense oligonucleotides can sometimes be designed to target and bypass or overcome a patient's genetic mutation, in particular those lesions that compromise normal pre-mRNA processing. Antisense oligonucleotides can alter gene expression through a variety of mechanisms as determined by the chemistry and antisense oligomer design. Through targeting the pre-mRNA, antisense oligonucleotides can alter splicing and induce a specific spliceoform or disrupt the reading frame, target an RNA transcript for degradation through RNaseH activation, block ribosome initiation of protein translation or disrupt miRNA function. The recent accelerated approval of eteplirsen (renamed Exondys 51™) by the Food and Drug Administration, for the treatment of Duchenne muscular dystrophy, and nusinersen, for the treatment of spinal muscular atrophy, herald a new and exciting era in splice-switching antisense oligonucleotide applications to treat inherited diseases. This review considers the potential of antisense oligonucleotides to treat inherited lung diseases of childhood with a focus on cystic fibrosis and disorders of surfactant protein metabolism.
Keywords: Antisense oligonucleotides; Childhood; Cystic fibrosis; Inherited diseases; Surfactant disorders.
Conflict of interest statement
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- FDA grants accelerated approval to first drug for Duchenne muscular dystrophy. U.S. Food and Drug Administration news release 19th September 2016; Available from: https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm521263.htm
-
- FDA approves first drug for spinal muscular atrophy. U.S. Food and Drug Administration news release 23 December 2016; Available from: https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm534611.htm
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

