Drosophila as a Model System for Neurotransmitter Measurements
- PMID: 29411967
- PMCID: PMC6093779
- DOI: 10.1021/acschemneuro.7b00456
Drosophila as a Model System for Neurotransmitter Measurements
Abstract
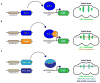
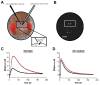
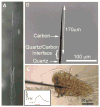
Drosophila melanogaster, the fruit fly, is an important, simple model organism for studying the effects of genetic mutations on neuronal activity and behavior. Biologists use Drosophila for neuroscience studies because of its genetic tractability, complex behaviors, well-known and simple neuroanatomy, and many orthologues to human genes. Neurochemical measurements in Drosophila are challenging due to the small size of the central nervous system. Recently, methods have been developed to measure real-time neurotransmitter release and clearance in both larvae and adults using electrochemistry. These studies have characterized dopamine, serotonin, and octopamine release in both wild type and genetic mutant flies. Tissue content measurements are also important, and separations are predominantly used. Capillary electrophoresis, with either electrochemical, laser-induced fluorescence, or mass spectrometry detection, facilitates tissue content measurements from single, isolated Drosophila brains or small samples of hemolymph. Neurochemical studies in Drosophila have revealed that flies have functioning transporters and autoreceptors, that their metabolism is different than in mammals, and that flies have regional, life stage, and sex differences in neurotransmission. Future studies will develop smaller electrodes, expand optical imaging techniques, explore physiological stimulations, and use advanced genetics to target single neuron release or study neurochemical changes in models of human diseases.
Keywords: Drosophila; capillary electrophoresis; dopamine; glutamate; octopamine; optogenetics; serotonin; voltammetry.
Figures

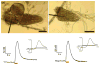

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

