Phosphorylation of the Amino Terminus of the Dopamine Transporter: Regulatory Mechanisms and Implications for Amphetamine Action
- PMID: 29413521
- PMCID: PMC6341980
- DOI: 10.1016/bs.apha.2017.09.002
Phosphorylation of the Amino Terminus of the Dopamine Transporter: Regulatory Mechanisms and Implications for Amphetamine Action
Abstract
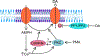
Amphetamines (AMPHs) are potent psychostimulants that are widely used and abused, with profound medical and societal impact. Their actions at dopaminergic neurons are thought to mediate their therapeutic efficacy as well as their liability for abuse and dependence. AMPHs target the dopamine transporter (DAT), the plasmalemmal membrane protein that mediates the inactivation of released dopamine (DA) through its reuptake. AMPHs act as substrates for DAT and are known to cause mobilization of dopamine (DA) to the cell exterior via DAT-mediated reverse transport (efflux). It has become increasingly evident that the mechanisms that regulate AMPH-induced DA efflux are distinct from those that regulate DA uptake. Central to these mechanisms is the phosphorylation of the DAT amino (N)-terminus, which has been repeatedly demonstrated to facilitate DAT-mediated DA efflux, without impacting other aspects of DAT physiology. This review aims to summarize the current status of knowledge regarding DAT N-terminal phosphorylation and its regulation by protein modulators and the membrane microenvironment. A better understanding of these mechanisms may lead to the identification of novel therapeutic approaches that interfere selectively with the pharmacological effects of AMPHs without altering the physiological function of DAT.
Keywords: Dopamine efflux; Kinases; Lipid rafts; Phosphatases; Psychostimulants; Vesicular monoamine transporters.
© 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
Figures
References
-
- Adkins EM, Samuvel DJ, Fog JU, Eriksen J, Jayanthi LD, Vaegter CB, et al. (2007). Membrane mobility and microdomain association of the dopamine transporter studied with fluorescence correlation spectroscopy and fluorescence recovery after photobleaching. Biochemistry, 46(37), 10484–10497. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

